Page 55 - Read Online
P. 55
Malherbe et al. Rare Dis Orphan Drugs J 2024;3:7 https://dx.doi.org/10.20517/rdodj.2023.49 Page 5 of 11
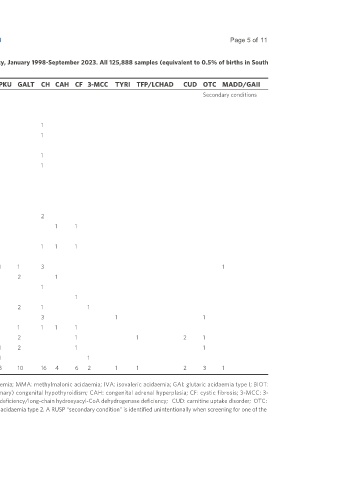
Table 1. Summary of biochemical NBS results, Centre for Metabolomics, North-West University, January 1998-September 2023. All 125,888 samples (equivalent to 0.5% of births in South
Africa) were screened using a panel of 22 conditions
year Project Total samples Total positive PA MMA IVA GAI BIOT PKU GALT CH CAH CF 3-MCC TYRI TFP/LCHAD CUD OTC MADD/GAII
RUSP core conditions Secondary conditions
1998 Government 445 0
1999 Government 4,903 0
2000 Government 8,410 1 1
2001 Government 10,674 1 1
2002 Government 8,886 0
2003 Government 11,399 1 1
2004 Government 12,665 1 1
2005 Government 11,933 0
2006 Government 3,121 0
2007 Private 106 0
2008 Private 1,325 0
2009 Private 1,163 3 1 2
2010 Private 871 3 1 1 1
2011 Private 811 0
2012 Private 951 5 1 1 1 1 1
2013 Private 1,770 1 1
2014 Private 2,897 9 1 1 + 1* 1 1 3 1
2015 Private 3,442 6 1 1 1 2 1
2016 Private 4,245 1 1
2017 Private 5,486 1 1
2018 Private 5,596 8 4 2 1 1
2019 Private 5,567 5 3 1 1
2020 Private 5,346 11 3 1 3 1 1 1 1
2021 Private 5,362 13 2 + 3* 1 2 1 1 2 1
2022 Private 4,702 5 1 2 1 1
2023 Private 3,812 5 3 1 1
Total 125,888 80 10 2 3 2 14 3 10 16 4 6 2 1 1 2 3 1
*Indicates cases unconfirmed via further review following initial positive screening. PA: Propionic acidaemia; MMA: methylmalonic acidaemia; IVA: isovaleric acidaemia; GAI: glutaric acidaemia type I; BIOT:
biotinidase deficiency; PKU: phenylketonuria; GALT: galactosaemia due to GALT deficiency; CH: (primary) congenital hypothyroidism; CAH: congenital adrenal hyperplasia; CF: cystic fibrosis; 3-MCC: 3-
methylcrotonyl-CoA carboxylase deficiency; TYRI: tyrosinemia type I; TFP/LCHAD: trifunctional protein deficiency/long-chain hydroxyacyl-CoA dehydrogenase deficiency; CUD: carnitine uptake disorder; OTC:
ornithine transcarbamylase deficiency; MADD/GAII: multiple acyl-CoA dehydrogenase deficiency/glutaric acidaemia type 2. A RUSP “secondary condition” is identified unintentionally when screening for one of the
core conditions; or as a consequence of confirmatory testing for an out-of-range result of a core condition.