Page 27 - Read Online
P. 27
Page 6 of 10 Berberoglu et al. Plast Aesthet Res 2024;11:14 https://dx.doi.org/10.20517/2347-9264.2023.101
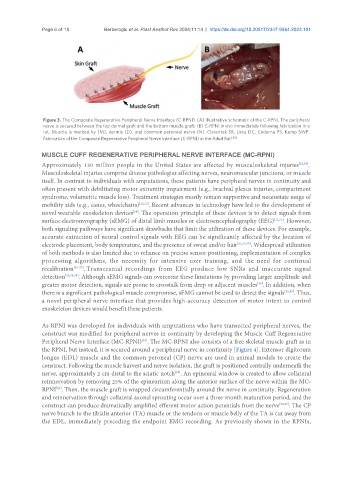
Figure 3. The Composite Regenerative Peripheral Nerve Interface (C-RPNI). (A) Illustrative schematic of the C-RPNI. The peripheral
nerve is secured between the top dermal graft and the bottom muscle graft; (B) C-RPNI in vivo immediately following fabrication in a
rat. Muscle is marked by (M), dermis (D), and common peroneal nerve (N). (Svientek SR, Ursu DC, Cederna PS, Kemp SWP.
Fabrication of the Composite Regenerative Peripheral Nerve Interface (C-RPNI) in the Adult Rat [30] .
MUSCLE CUFF REGENERATIVE PERIPHERAL NERVE INTERFACE (MC-RPNI)
Approximately 130 million people in the United States are affected by musculoskeletal injuries [32,33] .
Musculoskeletal injuries comprise diverse pathologies affecting nerves, neuromuscular junctions, or muscle
itself. In contrast to individuals with amputations, these patients have peripheral nerves in continuity and
often present with debilitating motor extremity impairment (e.g., brachial plexus injuries, compartment
syndrome, volumetric muscle loss). Treatment strategies mostly remain supportive and necessitate usage of
mobility aids (e.g., canes, wheelchairs) [32,33] . Recent advances in technology have led to the development of
[34]
novel wearable exoskeleton devices . The operation principle of these devices is to detect signals from
surface electromyography (sEMG) of distal limb muscles or electroencephalography (EEG) [32,33] . However,
both signaling pathways have significant drawbacks that limit the utilization of these devices. For example,
accurate extraction of neural control signals with EEG can be significantly affected by the location of
electrode placement, body temperature, and the presence of sweat and/or hair [32,33,35] . Widespread utilization
of both methods is also limited due to reliance on precise sensor positioning, implementation of complex
processing algorithms, the necessity for intensive user training, and the need for continual
recalibration [35-37] . Transcranial recordings from EEG produce low SNRs and inaccurate signal
detection [32,33,35] . Although sEMG signals can overcome these limitations by providing larger amplitude and
greater motor detection, signals are prone to crosstalk from deep or adjacent muscles . In addition, when
[38]
there is a significant pathological muscle compromise, sEMG cannot be used to detect the signals [32,33] . Thus,
a novel peripheral nerve interface that provides high-accuracy detection of motor intent to control
exoskeleton devices would benefit these patients.
As RPNI was developed for individuals with amputations who have transected peripheral nerves, the
construct was modified for peripheral nerves in continuity by developing the Muscle Cuff Regenerative
Peripheral Nerve Interface (MC-RPNI) . The MC-RPNI also consists of a free skeletal muscle graft as in
[33]
the RPNI, but instead, it is secured around a peripheral nerve in continuity [Figure 4]. Extensor digitorum
longus (EDL) muscle and the common peroneal (CP) nerve are used in animal models to create the
construct. Following the muscle harvest and nerve isolation, the graft is positioned centrally underneath the
[33]
nerve, approximately 2 cm distal to the sciatic notch . An epineural window is created to allow collateral
reinnervation by removing 25% of the epineurium along the anterior surface of the nerve within the MC-
RPNI . Then, the muscle graft is wrapped circumferentially around the nerve in continuity. Regeneration
[33]
and reinnervation through collateral axonal sprouting occur over a three-month maturation period, and the
construct can produce dramatically amplified efferent motor action potentials from the nerve [32,33] . The CP
nerve branch to the tibialis anterior (TA) muscle or the tendons or muscle belly of the TA is cut away from
the EDL, immediately preceding the endpoint EMG recording. As previously shown in the RPNIs,