Page 53 - Read Online
P. 53
Page 16 of 25 Bertolini et al. Plast Aesthet Res 2023;10:34 https://dx.doi.org/10.20517/2347-9264.2022.121
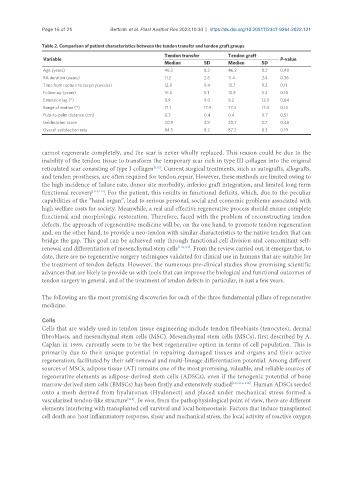
Table 2. Comparison of patient characteristics between the tendon transfer and tendon graft groups
Tendon transfer Tendon graft
Variable P-value
Median SD Median SD
Age (years) 46.3 8.3 46.2 8.2 0.40
RA duration (years) 11.2 2.8 11.4 2.4 0.36
Time from rupture to surgery(weeks) 12.8 9.4 13.7 9.2 0.11
Follow-up (years) 16.0 5.1 13.9 3.3 0.10
Extension lag (°) 8.9 9.0 8.2 12.0 0.84
Range of motion (°) 71.1 17.9 77.3 17.0 0.12
Pulp-to-palm distance (cm) 0.3 0.4 0.4 0.7 0.51
Geldmacher score 20.9 3.9 20.7 3.7 0.48
Overall satisfaction rate 84.5 8.2 87.2 8.3 0.19
cannot regenerate completely, and the scar is never wholly replaced. This reason could be due to the
inability of the tendon tissue to transform the temporary scar rich in type III collagen into the original
reticulated scar consisting of type I collagen . Current surgical treatments, such as autografts, allografts,
[112]
and tendon prostheses, are often required for tendon repair. However, these methods are limited owing to
the high incidence of failure rate, donor site morbidity, inferior graft integration, and limited long term
functional recovery [113,114] . For the patient, this results in functional deficits, which, due to the peculiar
capabilities of the “hand organ”, lead to serious personal, social and economic problems associated with
high welfare costs for society. Meanwhile, a real and effective regenerative process should ensure complete
functional and morphologic restoration. Therefore, faced with the problem of reconstructing tendon
defects, the approach of regenerative medicine will be, on the one hand, to promote tendon regeneration
and, on the other hand, to provide a neo-tendon with similar characteristics to the native tendon that can
bridge the gap. This goal can be achieved only through functional cell division and concomitant self-
renewal and differentiation of mesenchymal stem cells [110,115] . From the review carried out, it emerges that, to
date, there are no regenerative surgery techniques validated for clinical use in humans that are suitable for
the treatment of tendon defects. However, the numerous pre-clinical studies show promising scientific
advances that are likely to provide us with tools that can improve the biological and functional outcomes of
tendon surgery in general, and of the treatment of tendon defects in particular, in just a few years.
The following are the most promising discoveries for each of the three fundamental pillars of regenerative
medicine.
Cells
Cells that are widely used in tendon tissue engineering include tendon fibroblasts (tenocytes), dermal
fibroblasts, and mesenchymal stem cells (MSC). Mesenchymal stem cells (MSCs), first described by A.
Caplan in 1989, currently seem to be the best regenerative option in terms of cell population. This is
primarily due to their unique potential in repairing damaged tissues and organs and their active
regeneration, facilitated by their self-renewal and multi-lineage differentiation potential. Among different
sources of MSCs, adipose tissue (AT) remains one of the most promising, valuable, and reliable sources of
regenerative elements as adipose-derived stem cells (ADSCs), even if the tenogenic potential of bone
marrow-derived stem cells (BMSCs) has been firstly and extensively studied [110,116-118] . Human ADSCs seeded
onto a mesh derived from hyaluronan (Hyalonect) and placed under mechanical stress formed a
vascularized tendon-like structure . In vivo, from the pathophysiological point of view, there are different
[119]
elements interfering with transplanted cell survival and local homeostasis. Factors that induce transplanted
cell death are: host inflammatory response, shear and mechanical stress, the local activity of reactive oxygen