Page 555 - Read Online
P. 555
Maurina et al. Mini-invasive Surg 2021;5:53 https://dx.doi.org/10.20517/2574-1225.2021.88 Page 3 of 13
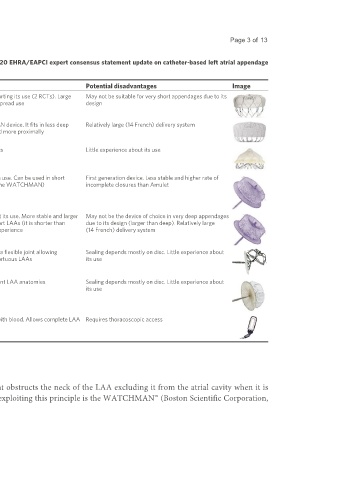
Table 1. Available left atrial appendage occlusion devices. Adapted and modified from the 2020 EHRA/EAPCI expert consensus statement update on catheter-based left atrial appendage
occlusion [12]
Device name Manufacturer Principle RCTs Advantages Potential disadvantages Image
[13]
WATCHMAN Boston Scientific Plug PROTECT-AF , Strong scientific evidence supporting its use (2 RCTs). Large May not be suitable for very short appendages due to its
[14]
PREVAIL clinical experience due to widespread use design
WATCHMAN Boston Scientific Plug None Second iteration of WATCHMAN device. It fits in less deep Relatively large (14 French) delivery system
FLX appendages and can be released more proximally
WaveCrest Biosense Plug None Can be used in short appendages Little experience about its use
Webster
Amplatzer Abbott Vascular Pacifier None Large registries documenting its use. Can be used in short First generation device. Less stable and higher rate of
Cardiac Plug appendages (it is shorter than the WATCHMAN) incomplete closures than Amulet
[15]
Amulet Abbott Vascular Pacifier AMULET-IDE Large registries and 1 RCT about its use. More stable and larger May not be the device of choice in very deep appendages
lobe than the ACP. Good for short LAAs (it is shorter than due to its design (larger than deep). Relatively large
WATCHMAN). Large clinical experience (14 French) delivery system
Ultraseal Cardia Inc. Pacifier None Disc and lobe are connected by a flexible joint allowing Sealing depends mostly on disc. Little experience about
orientation of the disc even in tortuous LAAs its use
LAmbre Lifetech Pacifier None Can be implanted in very different LAA anatomies Sealing depends mostly on disc. Little experience about
its use
ATRICLIP AtriCure Surgical None No foreign material in contact with blood. Allows complete LAA Requires thoracoscopic access
epicardial occlusion
exclusion
ACP: Amplatzer cardiac plug; RCT: randomized clinical trial; LAA: left atrial appendage.
The plug principle
Plugs are endovascular-delivered devices consisting of a lobe or umbrella that obstructs the neck of the LAA excluding it from the atrial cavity when it is
completely endothelialized. The first CE-approved (CE-mark in 2005) device exploiting this principle is the WATCHMAN™ (Boston Scientific Corporation,