Page 21 - Read Online
P. 21
Cidon. J Unexplored Med Data 2018;3:8 I http://dx.doi.org/10.20517/2572-8180.2018.03 Page 5 of 16
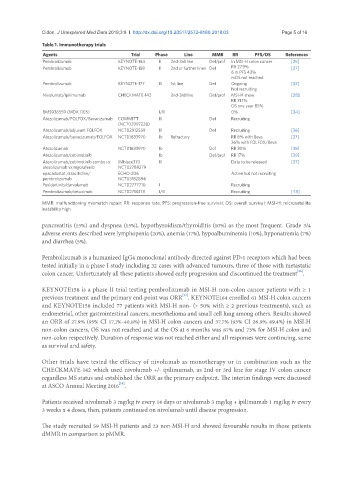
Table 1. Immunotherapy trials
Agents Trial Phase Line MMR RR PFS/OS References
Pembrolizumab KEYNOTE-164 II 2nd-3rd line Def/prof In MSI-H colon cancer [25]
Pembrolizumab KEYNOTE-158 II 2nd or further lines Def RR 27.9% [27]
6 m PFS 43%
mOS not reached
Pembrolizumab KEYNOTE-177 III 1st line Def Ongoing [32]
Not recruiting
Nivolumab/ipilimumab CHECKMATE-142 2nd-3rd line Def/prof MSI-H show [28]
RR 31.1%
OS one year 85%
BMS936559 (MDX 1105) I/II 0% [34]
Atezolizumab/FOLFOX/Bevacizumab COMMITT III Def Recruiting
(NCT02997228)
Atezolizumab/adjuvant FOLFOX NCT02912559 III Def Recruiting [36]
Atezolizumab/bevacizumab/FOLFOX NCT01633970 Ib Refractory RR 8% with Beva [37]
36% with FOLFOX/Beva
Atezolizumab NCT01633970 Ib Def RR 30% [38]
Atezolizumab/cobimetinib Ib Def/prof RR 17% [39]
Atezolizumab/cobimetinib combo vs. IMblaze370 III Data to be released [37]
atezolizumab vx regorafenib NCT02788279
epacadostat /azacitidine/ ECHO-206 Active but not recruiting
pembrolizumab NCT03182894
Pexidartinib/durvalumab NCT02777710 I Recruiting
Pembrolizumab/cetuximab NCT02713373 I/II Recruiting [43]
MMR: malfunctioning mismatch repair; RR: response rate; PFS: progression-free survival; OS: overall survival; MSI-H: microsatellite
instability high
pancreatitis (15%) and dyspnea (15%), hypothyroidism/thyroiditis (10%) as the most frequent. Grade 3/4
adverse events described were lymphopenia (20%), anemia (17%), hypoalbuminemia (10%), hyponatremia (7%)
and diarrhea (5%).
Pembrolizumab is a humanized IgG4 monoclonal antibody directed against PD-1 receptors which had been
tested initially in a phase I study including 32 cases with advanced tumours, three of those with metastatic
[26]
colon cancer. Unfortunately all these patients showed early progression and discontinued the treatment .
KEYNOTE158 is a phase II trial testing pembrolizumab in MSI-H non-colon cancer patients with ≥ 1
[27]
previous treatment and the primary end-point was ORR . KEYNOTE164 enrolled 61 MSI-H colon cancers
and KEYNOTE158 included 77 patients with MSI-H non- (> 50% with ≥ 2 previous treatments), such as
endometrial, other gastrointestinal cancers, mesothelioma and small cell lung among others. Results showed
an ORR of 27.9% (95% CI 17.1%-40.8%) in MSI-H colon cancers and 37.7% (95% CI 26.9%-49.4%) in MSI-H
non-colon cancers, OS was not reached and at the OS at 6 months was 87% and 73% for MSI-H colon and
non-colon respectively. Duration of response was not reached either and all responses were continuing, same
as survival and safety.
Other trials have tested the efficacy of nivolumab as monotherapy or in combination such as the
CHECKMATE-142 which used nivolumab +/- ipilimumab, as 2nd or 3rd line for stage IV colon cancer
regardless MS status and established the ORR as the primary endpoint. The interim findings were discussed
[28]
at ASCO Annual Meeting 2016 .
Patients received nivolumab 3 mg/kg iv every 14 days or nivolumab 3 mg/kg + ipilimumab 1 mg/kg iv every
3 weeks x 4 doses, then, patients continued on nivolumab until disease progression.
The study recruited 59 MSI-H patients and 23 non-MSI-H and showed favourable results in those patients
dMMR in comparison to pMMR.