Page 438 - Read Online
P. 438
Safa. J Cancer Metastasis Treat 2020;6:36 I http://dx.doi.org/10.20517/2394-4722.2020.55 Page 5 of 15
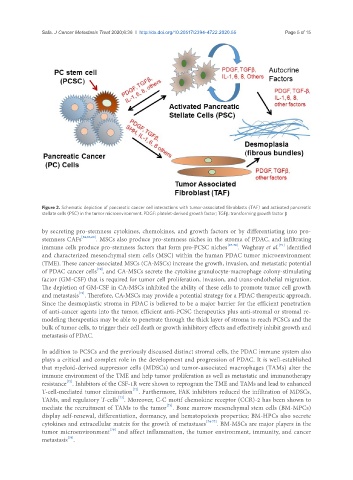
Figure 2. Schematic depiction of pancreatic cancer cell interactions with tumor-associated fibroblasts (TAF) and activated pancreatic
stellate cells (PSC) in the tumor microenvironment. PDGF: platelet-derived growth factor; TGFβ: transforming growth factor β
by secreting pro-stemness cytokines, chemokines, and growth factors or by differentiating into pro-
stemness CAFs [62,63,66] . MSCs also produce pro-stemness niches in the stroma of PDAC, and infiltrating
[71]
immune cells produce pro-stemness factors that form pro-PCSC niches [67-70] . Waghray et al. identified
and characterized mesenchymal stem cells (MSC) within the human PDAC tumor microenvironment
(TME). These cancer-associated MSCs (CA-MSCs) increase the growth, invasion, and metastatic potential
[71]
of PDAC cancer cells , and CA-MSCs secrete the cytokine granulocyte-macrophage colony-stimulating
factor (GM-CSF) that is required for tumor cell proliferation, invasion, and trans-endothelial migration.
The depletion of GM-CSF in CA-MSCs inhibited the ability of these cells to promote tumor cell growth
[71]
and metastasis . Therefore, CA-MSCs may provide a potential strategy for a PDAC therapeutic approach.
Since the desmoplastic stroma in PDAC is believed to be a major barrier for the efficient penetration
of anti-cancer agents into the tumor, efficient anti-PCSC therapeutics plus anti-stromal or stromal re-
modeling therapeutics may be able to penetrate through the thick layer of stroma to reach PCSCs and the
bulk of tumor cells, to trigger their cell death or growth inhibitory effects and effectively inhibit growth and
metastasis of PDAC.
In addition to PCSCs and the previously discussed distinct stromal cells, the PDAC immune system also
plays a critical and complex role in the development and progression of PDAC. It is well-established
that myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) alter the
immune environment of the TME and help tumor proliferation as well as metastatic and immunotherapy
[72]
resistance . Inhibitors of the CSF-1R were shown to reprogram the TME and TAMs and lead to enhanced
[72]
T-cell-mediated tumor elimination . Furthermore, FAK inhibitors reduced the infiltration of MDSCs,
[72]
TAMs, and regulatory T-cells . Moreover, C-C motif chemokine receptor (CCR)-2 has been shown to
[73]
mediate the recruitment of TAMs to the tumor . Bone marrow mesenchymal stem cells (BM‐MPCs)
display self‐renewal, differentiation, dormancy, and hematopoiesis properties; BM‐HPCs also secrete
cytokines and extracellular matrix for the growth of metastases [74-77] . BM‐MSCs are major players in the
[78]
tumor microenvironment and affect inflammation, the tumor environment, immunity, and cancer
[79]
metastasis .