Page 405 - Read Online
P. 405
D'Angelo et al. J Cancer Metastasis Treat 2019;5:30 I http://dx.doi.org/10.20517/2394-4722.2018.86 Page 11 of 18
A B
C D
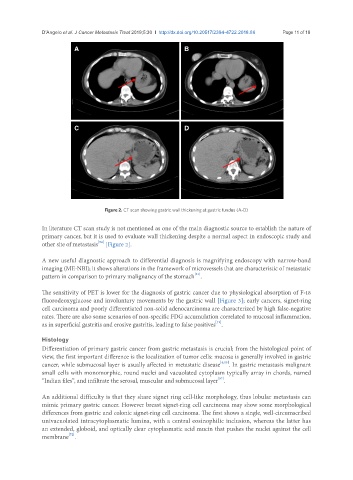
Figure 2. CT scan showing gastric wall thickening at gastric fundus (A-D)
In literature CT scan study is not mentioned as one of the main diagnostic source to establish the nature of
primary cancer, but it is used to evaluate wall thickening despite a normal aspect in endoscopic study and
[54]
other site of metastasis [Figure 2].
A new useful diagnostic approach to differential diagnosis is magnifying endoscopy with narrow-band
imaging (ME-NBI); it shows alterations in the framework of microvessels that are characteristic of metastatic
[51]
pattern in comparison to primary malignancy of the stomach .
The sensitivity of PET is lower for the diagnosis of gastric cancer due to physiological absorption of F-18
fluorodeoxyglucose and involuntary movements by the gastric wall [Figure 3]; early cancers, signet-ring
cell carcinoma and poorly differentiated non-solid adenocarcinoma are characterized by high false-negative
rates. There are also some scenarios of non-specific FDG accumulation correlated to mucosal inflammation,
[71]
as in superficial gastritis and erosive gastritis, leading to false positives .
Histology
Differentiation of primary gastric cancer from gastric metastasis is crucial; from the histological point of
view, the first important difference is the localization of tumor cells: mucosa is generally involved in gastric
cancer, while submucosal layer is usually affected in metastatic disease [4,15] . In gastric metastasis malignant
small cells with monomorphic, round nuclei and vacuolated cytoplasm typically array in chords, named
[57]
“Indian files”, and infiltrate the serosal, muscular and submucosal layer .
An additional difficulty is that they share signet ring cell-like morphology, thus lobular metastasis can
mimic primary gastric cancer. However breast signet-ring cell carcinoma may show some morphological
differences from gastric and colonic signet-ring cell carcinoma. The first shows a single, well-circumscribed
univacuolated intracytoplasmatic lumina, with a central eosinophilic inclusion, whereas the latter has
an extended, globoid, and optically clear cytoplasmatic acid mucin that pushes the nuclei against the cell
[72]
membrane .