Page 64 - Read Online
P. 64
Torres et al. J Cancer Metastasis Treat 2018;4:4 I http://dx.doi.org/10.20517/2394-4722.2017.49 Page 11 of 25
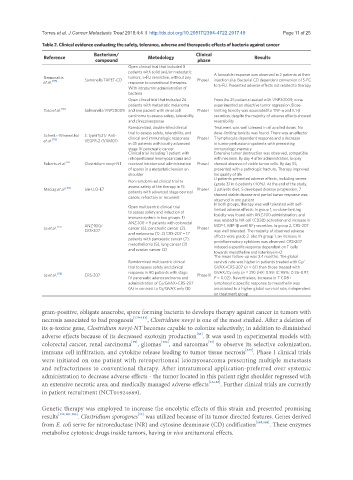
Table 2. Clinical evidence evaluating the safety, tolerance, adverse and therapeutic effects of bacteria against cancer
Reference Bacterium/ Metodology Clinical Results
compound
phase
Open clinical trial that included 3
patients with solid and/or metastatic
Nemunaitis tumors, 5-FU sensistive, without any A favorable response was observed in 2 patients at their
et al. [175] Samonella TAPET-CD response to coventional therapies. Phase I injection site. Bacterial CD dependent conversion of 5-FC
With intratumor administration of to 5-FU. Presented adverse effects not related to therapy
bacteria
Open clincal trial that included 24 From the 25 patients treated with VNP20009, none
patients with metastatic melanoma experimented an objective tumor regression. Dose-
Toso et al. [110] Salmonella VNP20009. and one pacient with renal cell Phase I limiting toxicity was associated to TNF-α and IL1-β
carcinoma to assess safety, tolerability secretion, despite the majority of adverse effects showed
and clinical response reversibility
Ramdomized, double-blind clinical Treatment was well toleraed in all applied doses. No
trial to assess safety, tolerability, and dose-limiting toxicity was found. There was an effector
Schmitz-Winnenthal S. typhiTy21/ Anti-
et al. [176] VEGFR-2 (VXM01) clinical and immunologic responses Phase I T lymphocyte dependent response and a decrease
in 45 patients with locally advanced in tumor perfussion in patients with preexisting
stage IV pancreatic cancer immunologic memory
Clinical trial including 1 patient with Extensive tumor destruction was observed, compatible
retroperitoneal leiomyosarcoma and with necrosis. By day 4 after administration, biopsy
Roberts et al. [13] Clostridium novyi-NT received intratumoral administration Phase I showed absence of viable tumor cells. By day 55,
of spores in a metastatic lession on presented with a pathologic fracture. Therapy improved
shoulder his quality of life
Ll patients presented adverse effects, including severe
Non ramdomized clinical trial to
assess safety of the therapy in 15 (grade 3) in 6 patients (40%). At the end of the study,
Maciag et al. [185] Lm-LLO-E7 Phase I 2 patients died, 5 developed disease progression, 7
patients with advanced stage cevrical
cancer, refractary or recurrent showed stable disease and partial tumor response was
observed in one patient
In both groups, therapy was well tolerated with self-
Open multicentric clinical trial
to assess safety and induction of limited adverse effects. In group 1, no dose-limiting
immune system in two groups: 1) toxicity was found with ANZ-100 administration, and
ANZ-100 = 9 patients with colorectal was related to NK cell (CD38) activation and increase in
Le et al. [177] ANZ-100/ cancer (6), pancreatic cancer (2), Phase I MCP-1, MIP-1β and INFγ secretion. In group 2, CRS-207
CRS-207 was well tolerated. The majority of observed adverse
and melanoma (1). 2) CRS-207 = 17
patients with pancreatic cancer (7), effects were grade 2. Like IN group 1, an increase in
mesothelioma (5), lung cancer (3) proinflammatory cytokines was observed. CRS-207
and ovarian cancer (2) induced a specific response dependent on T cells
towards mesotheline and listeriolysin-O
The mean follow-up was 3.4 months. The global
Ramdomized multicentric clinical survival rate was higher in patients treated with Cy/
trial to assess safety and clinical GVAX+CRS-207 (n = 61) than those treated with
response in 90 patients with stage GVAX/Cy only (n = 29) (HR: 0.59; IC 95%: 0.36-0.97,
Le et al. [178] CRS-207 Phase II
IV pancreatic adenocarcinoma and P = 0.02). Nevertheless, increase in T CD8+
administration of Cy/GVAX+CRS-207 lymphocytic specific response to mesothelin was
(A) in contrast to Cy/GVAX only (B) associated to a higher global survival rate, independent
on treatment group
gram-positive, obligate anaerobe, spore forming bacteria to developa therapy against cancer in tumors with
necrosis associated to bad prognosis [179-181] , Clostridium novyi is one of the most studied. After a deletion of
its α-toxine gene, Clostridium novyi-NT becomes capable to colonize selectively; in addition to diminished
[82]
adverse effects because of its decreased exotoxin production . It was used in experimental models with
[13]
[99]
colorectal cancer, renal carcinoma , gliomas [182] , and sarcomas to observe its selective colonization,
immune cell infiltration, and cytokine release leading to tumor tissue necrosis [125] . Phase I clinical trials
were initiated on one patient with retroperitoneal leiomyosarcoma presenting multiple metastasis
and refractoriness to conventional therapy. After intratumoral application-preferred over systemic
administration to decrease adverse effects - the tumor located in this patient right shoulder regressed with
an extensive necrotic area; and medically managed adverse effects [13,183] . Further clinical trials are currently
in patient recruitment (NCT01924689).
Genetic therapy was employed to increase the oncolytic effects of this strain and presented promising
[13]
results [122,184,185] . Clostridium sporogenes was utilized because of its tumor directed features. Genes derived
from E. coli serve for nitroreductase (NR) and cytosine deaminase (CD) codification [185,186] . These enzymes
metabolize cytotoxic drugs inside tumors, having in vivo antitumoral effects.