Page 595 - Read Online
P. 595
Ralph et al. J Cancer Metastasis Treat 2018;4:49 I http://dx.doi.org/10.20517/2394-4722.2018.42 Page 15 of 26
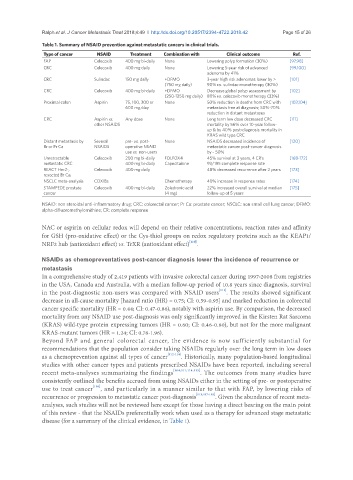
Table 1. Summary of NSAID prevention against metastatic cancers in clinical trials.
Type of cancer NSAID Treatment Combination with Clinical outcome Ref.
FAP Celecoxib 400 mg bi-daily None Lowering polyp formation (30%) [97,98]
CRC Celecoxib 400 mg daily None Lowering 5-year risk of advanced [99,100]
adenoma by 41%
CRC Sulindac 150 mg daily +DFMO 3-year high risk adenomas lower by > [101]
(750 mg daily) 90% vs. sulindac monotherapy (30%)
CRC Celecoxib 400 mg bi-daily +DFMO Decrease global polyp assessment by [102]
(250-1250 mg daily) 80% vs. celecoxib monotherapy (33%)
Proximal colon Aspirin 75, 100, 300 or None 50% reduction in deaths from CRC with [103,104]
600 mg/day metastasis free at diagnosis; 50%-70%
reduction in distant metastases
CRC Aspirin vs. Any dose None Long term low dose decreased CRC [111]
other NSAIDS mortality by 56% over 10-year follow-
up & by 40% post-diagnosis mortality in
KRAS wild type CRC
Distant metastasis by Several pre- vs. post- None NSAIDS decreased incidence of [120]
Br or Pr Ca NSAIDS operative NSAID metastatic cancer post-cancer diagnosis
use vs. non-users by ~ 50%
Unretractable Celecoxib 200 mg bi-daily FOLFOX4 45% survival at 3 years, 4 CR’s [168-172]
metastatic CRC 400 mg bi-daily Capecitabine 93/195 complete response rate
REACT Her2-, Celecoxib 400 mg daily 48% decreased recurrence after 2 years [173]
resected Br Ca.
NSCLC meta-analysis COXIBs Chemotherapy 40% increase in response rates [174]
STAMPEDE prostate Celecoxib 400 mg bi-daily Zoledronic acid 22% increased overall survival at median [175]
cancer (4 mg) follow-up of 5 years
NSAID: non steroidal anti-inflammatory drug; CRC: colorectal cancer; Pr Ca: prostate cancer; NSCLC: non small cell lung cancer; DFMO:
alpha-difluoromethylornithine; CR: complete response
NAC or aspirin on cellular redox will depend on their relative concentrations, reaction rates and affinity
for GSH (pro-oxidative effect) or the Cys-thiol groups on redox regulatory proteins such as the KEAP1/
[110]
NRF2 hub (antioxidant effect) vs. TrXR (antioxidant effect) .
NSAIDs as chemopreventatives post-cancer diagnosis lower the incidence of recurrence or
metastasis
In a comprehensive study of 2,419 patients with invasive colorectal cancer during 1997-2008 from registries
in the USA, Canada and Australia, with a median follow-up period of 10.8 years since diagnosis, survival
[111]
in the post-diagnostic non-users was compared with NSAID users . The results showed significant
decrease in all-cause mortality [hazard ratio (HR) = 0.75; CI: 0.59-0.95] and marked reduction in colorectal
cancer specific mortality (HR = 0.44; CI: 0.47-0.86), notably with aspirin use. By comparison, the decreased
mortality from any NSAID use post-diagnosis was only significantly improved in the Kirsten Rat Sarcoma
(KRAS) wild-type protein expressing tumors (HR = 0.60; CI: 0.46-0.80), but not for the more malignant
KRAS-mutant tumors (HR = 1.24; CI: 0.78-1.96).
Beyond FAP and general colorectal cancer, the evidence is now sufficiently substantial for
recommendations that the population consider taking NSAIDs regularly over the long term in low doses
as a chemoprevention against all types of cancer [112-114] . Historically, many population-based longitudinal
studies with other cancer types and patients prescribed NSAIDs have been reported, including several
recent meta-analyses summarizing the findings [104,111,114,115] . The outcomes from many studies have
consistently outlined the benefits accrued from using NSAIDs either in the setting of pre- or postoperative
use to treat cancer [116] , and particularly in a manner similar to that with FAP, by lowering risks of
recurrence or progression to metastatic cancer post-diagnosis [113,117-119] . Given the abundance of recent meta-
analyses, such studies will not be reviewed here except for those having a direct bearing on the main point
of this review - that the NSAIDs preferentially work when used as a therapy for advanced stage metastatic
disease (for a summary of the clinical evidence, in Table 1).