Page 242 - Read Online
P. 242
Page 6 of 10 Harada et al. J Cancer Metastasis Treat 2018;4:18 I http://dx.doi.org/10.20517/2394-4722.2017.74
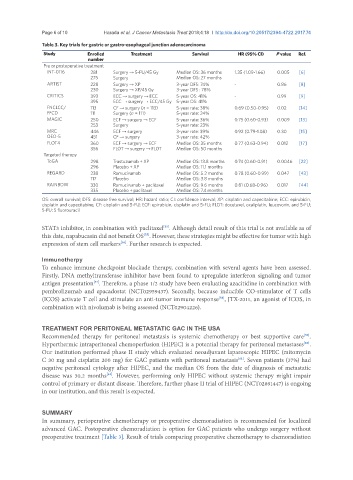
Table 3. Key trials for gastric or gastro-esophageal junction adenocarcinoma
Study Enrolled Treatment Survival HR (95% CI) P value Ref.
number
Pre or postoperative treatment
INT-0116 281 Surgery → 5-FU/45 Gy Median OS: 36 months 1.35 (1.09-1.66) 0.005 [6]
275 Surgery Median OS: 27 months
ARTIST 228 Surgery → XP 3-year DFS: 74% - 0.86 [8]
230 Surgery → XP/45 Gy 3-year DFS : 78%
CRITICS 393 ECC → surgery → ECC 5-year OS: 41% - 0.99 [9]
395 ECC → surgery → ECC/45 Gy 5-year OS: 41%
FNCLCC/ 113 CF → surgery (n = 113) 5-year rate: 38% 0.69 (0.50-0.95) 0.02 [14]
FFCD 111 Surgery (n = 111) 5-year rate: 24%
MAGIC 250 ECF → surgery → ECF 5-year rate: 36% 0.75 (0.60-0.93) 0.009 [13]
253 Surgery 5-year rate: 23%
MRC 446 ECF → surgery 3-year rate: 39% 0.92 (0.79-1.08) 0.30 [15]
OEO-5 451 CF → surgery 3-year rate: 42%
FLOT4 360 ECF → surgery → ECF Median OS: 35 months 0.77 (0.63-0.94) 0.012 [17]
356 FLOT → surgery → FLOT Median OS: 50 months
Targeted therapy
ToGA 298 Trastuzumab + XP Median OS: 13.8 months 0.74 (0.60-0.91) 0.0046 [22]
296 Placebo + XP Median OS: 11.1 months
REGARD 238 Ramucirumab Median OS: 5.2 months 0.78 (0.60-0.99) 0.047 [43]
117 Placebo Median OS: 3.8 months
RAINBOW 330 Ramucirumab + paclitaxel Median OS: 9.6 months 0.81 (0.68-0.96) 0.017 [44]
335 Placebo + paclitaxel Median OS: 7.4 months
OS: overall survival; DFS: disease free survival; HR: hazard ratio; CI: confidence interval; XP: cisplatin and capecitabine; ECC: epirubicin,
cisplatin and capecitabine; CF: cisplatin and 5-FU; ECF: epirubicin, cisplatin and 5-FU; FLOT: docetaxel, oxaliplatin, leucovorin, and 5-FU;
5-FU: 5 fluorouracil
STAT3 inhibitor, in combination with paclitaxel . Although detail result of this trial is not available as of
[55]
this date, napabucasin did not benefit OS . However, these strategies might be effective for tumor with high
[55]
expression of stem cell markers . Further research is expected.
[56]
Immunotherpy
To enhance immune checkpoint blockade therapy, combination with several agents have been assessed.
Firstly, DNA methyltransferase inhibitor have been found to upregulate interferon signaling and tumor
antigen presentation . Therefore, a phase 1/2 study have been evaluating azacitidine in combination with
[57]
pembrolizumab and epacadostat (NCT02959437). Secondly, because inducible CO-stimulator of T cells
(ICOS) activate T cell and stimulate an anti-tumor immune response , JTX-2011, an agonist of ICOS, in
[58]
combination with nivolumab is being assessed (NCT02904226).
TREATMENT FOR PERITONEAL METASTATIC GAC IN THE USA
[59]
Recommended therapy for peritoneal metastasis is systemic chemotherapy or best supportive care .
Hyperthermic intraperitoneal chemoperfusion (HIPEC) is a potential therapy for peritoneal metastases .
[60]
Our institution performed phase II study which evaluated neoadjuvant laparoscopic HIPEC (mitomycin
C 30 mg and cisplatin 200 mg) for GAC patients with peritoneal metastasis . Seven patients (37%) had
[61]
negative peritoneal cytology after HIPEC, and the median OS from the date of diagnosis of metastatic
disease was 30.2 months . However, performing only HIPEC without systemic therapy might impair
[61]
control of primary or distant disease. Therefore, further phase II trial of HIPEC (NCT02891447) is ongoing
in our institution, and this result is expected.
SUMMARY
In summary, perioperative chemotherapy or preoperative chemoradiation is recommended for localized
advanced GAC. Postoperative chemoradiation is option for GAC patients who undergo surgery without
preoperative treatment [Table 3]. Result of trials comparing preoperative chemotherapy to chemoradiation