Page 158 - Read Online
P. 158
Battaglin et al. J Cancer Metastasis Treat 2018;4:12 I http://dx.doi.org/10.20517/2394-4722.2018.04 Page 3 of 25
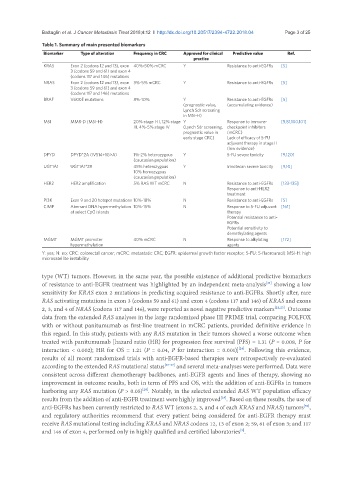
Table 1. Summary of main presented biomarkers
Biomarker Type of alteration Frequency in CRC Approved for clinical Predictive value Ref.
practice
KRAS Exon 2 (codons 12 and 13), exon 40%-50% mCRC Y Resistance to anti-EGFRs [5]
3 (codons 59 and 61) and exon 4
(codons 117 and 146) mutations
NRAS Exon 2 (codons 12 and 13), exon 3%-5% mCRC Y Resistance to anti-EGFRs [5]
3 (codons 59 and 61) and exon 4
(codons 117 and 146) mutations
BRAF V600E mutations 8%-10% Y Resistance to anti-EGFRs [5]
(prognostic value, (accumulating evidence)
Lynch Sdr screening
in MSI-H)
MSI MMR-D (MSI-H) 20% stage I-II, 12% stage Y Response to immune- [5,81,100,101]
III, 4%-5% stage IV (Lynch Sdr screening, checkpoint inhibitors
prognostic value in (mCRC)
early stage CRC) Lack of efficacy of 5-FU
adjuvant therapy in stage II
(low evidence)
DPYD DPYD*2A (IVS14+1G>A) 1%-2% heterozygous Y 5-FU severe toxicity [9,120]
(caucasian population)
UGT1A1 UGT1A1*28 45% heterozygous Y Irinotecan severe toxicity [9,10]
10% homozygous
(caucasian population)
HER2 HER2 amplification 5% RAS WT mCRC N Resistance to anti-EGFRs [133-135]
Response to anti-HER2
treatment
PI3K Exon 9 and 20 hotspot mutations 10%-18% N Resistance to anti-EGFRs [5]
CIMP Aberrant DNA hypermethylation 10%-15% N Response to 5-FU adjuvant [161]
at select CpG islands therapy
Potential resistance to anti-
EGFRs
Potential sensitivity to
demethylating agents
MGMT MGMT promoter 40% mCRC N Response to alkylating [172]
hypermethylation agents
Y: yes; N: no; CRC: colorectal cancer; mCRC: metastatic CRC; EGFR: epidermal growth factor receptor; 5-FU: 5-fluorouracil; MSI-H: high
microsatellite instability
type (WT) tumors. However, in the same year, the possible existence of additional predictive biomarkers
of resistance to anti-EGFR treatment was highlighted by an independent meta-analysis showing a low
[21]
sensitivity for KRAS exon 2 mutations in predicting acquired resistance to anti-EGFRs. Shortly after, rare
RAS activating mutations in exon 3 (codons 59 and 61) and exon 4 (codons 117 and 146) of KRAS and exons
2, 3, and 4 of NRAS (codons 117 and 146), were reported as novel negative predictive markers [22,23] . Outcome
data from the extended RAS analyses in the large randomized phase III PRIME trial, comparing FOLFOX
with or without panitumumab as first-line treatment in mCRC patients, provided definitive evidence in
this regard. In this study, patients with any RAS mutation in their tumors showed a worse outcome when
treated with panitumumab [hazard ratio (HR) for progression free survival (PFS) = 1.31 (P = 0.008, P for
interaction < 0.002); HR for OS = 1.21 (P = 0.04, P for interaction = 0.001)] . Following this evidence,
[24]
results of all recent randomized trials with anti-EGFR-based therapies were retrospectively re-evaluated
according to the extended RAS mutational status [25-27] and several meta-analyses were performed. Data were
consistent across different chemotherapy backbones, anti-EGFR agents and lines of therapy, showing no
improvement in outcome results, both in term of PFS and OS, with the addition of anti-EGFRs in tumors
harboring any RAS mutation (P > 0.05) . Notably, in the selected extended RAS WT population efficacy
[28]
results from the addition of anti-EGFR treatment were highly improved . Based on these results, the use of
[29]
anti-EGFRs has been currently restricted to RAS WT (exons 2, 3, and 4 of each KRAS and NRAS) tumors ,
[30]
and regulatory authorities recommend that every patient being considered for anti-EGFR therapy must
receive RAS mutational testing including KRAS and NRAS codons 12, 13 of exon 2; 59, 61 of exon 3; and 117
and 146 of exon 4, performed only in highly qualified and certified laboratories .
[5]