Page 18 - Read Online
P. 18
Lo et al. J Cancer Metastasis Treat 2022;8:30 https://dx.doi.org/10.20517/2394-4722.2022.48 Page 3 of 12
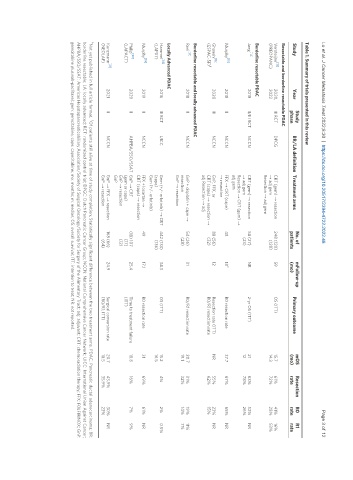
Table 1. Summary of trials presented in this review
Study No. of mFollow-up mOS Resection R0 R1
Study Year BR/LA definition Treatment arms Primary outcome
phase patients (mo) (mo) rate rate rate
Resectable and borderline resectable PDAC
Versteijne [13] 2020, III RCT DPCG CRT (gem) → resection 248 (120) 59 OS (ITT) 15.7 61% 41% 16%
(PREOPANC) 2022 → adj gem (128) 14.3 72% 28% 53%
Resection → adj gem
Borderline resectable PDAC
[14]
Jang 2018 II/III RCT NCCN CRT (gem) → resection 58 (27) NR 2-yr OS (ITT) 21 63% 52% NR
→ adj gem (23) 12 78% 26%
Resection → CRT (gem) →
adj gem
Murphy [15] 2018 II NCCN FFX → CRT (cape) 48 18 b R0 resection rate 37.7 67% 65% NR
→ resection
Ghaneh [16] 2020 III NCCN GnP, FFX, or 88 (56) 12 Resection rate (ITT) NR 55% 23% NR
a
(ESPAC-5F) CRT (cape) → resection → (32) R0/R1 resection rate 62% 15%
adj Resection → adj
Borderline resectable and locally advanced PDAC
[17]
Reni 2018 II NCCN GnP + cisplatin + cape → 54 (26) 31 R0/R1 resection rate 20.7 31% 19% 11%
resection (28) 19.1 32% 14% 7%
GnP → resection
Locally Advanced PDAC
[18]
Hammel 2016 III RCT UICC Gem (+/- erlotinib) → CRT 442 (133) 34.3 OS (ITT) 15.2 4% 2% 0.5%
(LAP07) (cape) (136) 16.5
Gem (+/- erlotinib)
[19]
Murphy 2019 II NCCN FFX + losartan → 49 17.1 R0 resection rate 31 69% 61% NR
CRT (cape) → resection
[20]
Philip 2020 II AHPBA/SSO/SSAT GnP → CRT (18) 107 25.4 Time to treatment failure 18.8 16% 7% 9%
(LAPACT) (gem or cape) (17) (ITT)
GnP → resection (12)
GnP
Kunzmann [21] 2021 II NCCN GnP → FFX → resection 168 (66) 24.9 Surgical conversion rate 20.7 43.9% 30% NR
(NEOLAP) GnP → resection (64) (R0/R1; ITT) 18.5 35.9% 23%
a b
Not yet published in full article format, Of patients still alive at time of study completion; Statistically significant difference between the two treatment arms. PDAC: Pancreatic ductal adenocarcinoma; BR:
borderline resectable; LA: locally advanced; RCT: randomized control trial; DPGC: Dutch Pancreatic Cancer Group; NCCN: National Comprehensive Cancer Network; UICC: International Union Against Cancer;
AHPBA/SSO/SSAT: Americas Hepatopancreaticobiliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract; adj: adjuvant; CRT: chemoradiation therapy; FFX: FOLFIRINOX; GnP:
gemcitabine plus nab-paclitaxel; gem: gemcitabine; cape: capecitabine; mo: months; m: median; OS: overall survival; ITT: intention to treat; NR: not reported.