Page 57 - Read Online
P. 57
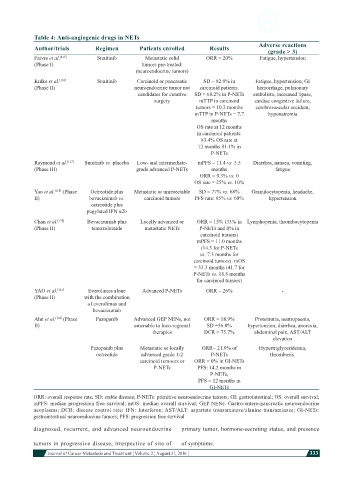
Table 4: Anti-angiogenic drugs in NETs
Author/trials Regimen Patients enrolled Results Adverse reactions
(grade > 3)
Faivre et al. [115] Sunitinib Metastatic solid ORR = 20% Fatigue, hypertension
(Phase I) tumors pre-treated:
(neuroendocrine tumors)
Kulke et al. [116] Sunitinib Carcinoid or pancreatic SD = 82.9% in Fatigue, hypertension, GI
(Phase II) neuroendocrine tumor not carcinoid patients. hemorrhage, pulmonary
candidates for curative SD = 68.2% in P-NETs embolism, increased lipase,
surgery mTTP in carcinoid cardiac congestive failure,
tumors = 10.2 months cerebrovascular accident,
mTTP in P-NETs = 7.7 hyponatremia
months
OS rate at 12 months
in carcinoid patients:
83.4% OS rate at
12 months 81.1% in
P-NETs
Raymond et al. [117] Sunitinib vs. placebo Low- and intermediate- mPFS = 11.4 vs. 5.5 Diarrhea, nausea, vomiting,
(Phase III) grade advanced P-NETs months fatigue
ORR = 9.3% vs. 0
OS rate = 25% vs. 10%
Yao et al. [118] (Phase Octreotide plus Metastatic or unresectable SD = 77% vs. 68% Granulocytopenia, headache,
II) bevacizumab vs. carcinoid tumors PFS rate: 95% vs. 68% hypertension
octreotide plus
pegylated IFN α2b
Chan et al. [120] Bevacizumab plus Locally advanced or ORR = 15% (33% in Lymphopenia, thrombocytopenia
(Phase II) temozolomide metastatic NETs P-NETs and 0% in
carcinoid tumors)
mPFS = 11.0 months
(14.3 for P-NETs
vs. 7.3 months for
carcinoid tumors). mOS
= 33.3 months (41.7 for
P-NETs vs. 18.8 months
for carcinoid tumors)
YAO et al. [121] Everolimus alone Advanced P-NETs ORR = 26% -
(Phase II) with the combination
of everolimus and
bevacizumab
Ahn et al. [123] (Phase Pazopanib Advanced GEP NENs, not ORR = 18.9% Proteinuria, neutropaenia,
II) amenable to loco-regional SD =56.8% hypertension, diarrhea, anorexia,
therapies DCR = 75.7% abdominal pain, AST/ALT
elevation
Pazopanib plus Metastatic or locally ORR= 21.9% of Hypertriglyceridemia,
octreotide advanced grade 1-2 P-NETs thrombosis.
carcinoid tumours or ORR = 0% in GI-NETs
P-NETs PFS: 14.2 months in
P-NETs,
PFS = 12 months in
GI-NETs
ORR: overall response rate; SD: stable disease; P-NETs: primitive neuroendocrine tumors; GI: gastrointestinal; OS: overall survival;
mPFS: median progression free survival; mOS: median overall survival; GEP NENs: Gastro-entero-pancreatic neuroendocrine
neoplasms; DCR: disease control rate; IFN: Interferon; AST/ALT: aspartate transaminase/alanine transaminase; GI-NETs:
gastrointestinal neuroendocrine tumors; PFS: progression free survival
diagnosed, recurrent, and advanced neuroendocrine primary tumor, hormone-secreting status, and presence
tumors in progressive disease, irrespective of site of of symptoms.
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ August 31, 2016 ¦ 333