Page 33 - Read Online
P. 33
D'Souza et al. J Cancer Metastasis Treat 2022;8:28 https://dx.doi.org/10.20517/2394-4722.2022.51 Page 5 of 15
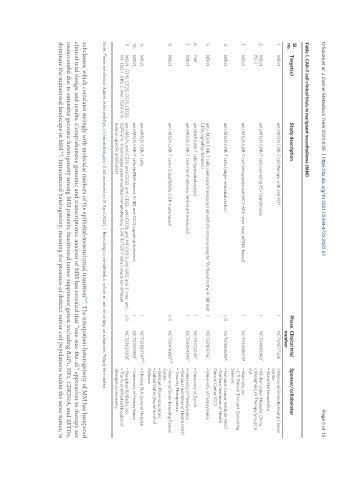
Table 1. CAR-T cell clinical trials in malignant mesothelioma (MM)
Sl.
no. Target(s) Study description Phase Clinical trial Sponsor/collaborator
number
1. MSLN anti-MSLN CAR-T cell therapy with anti-PD1 I NCT04577326 r • Memorial Sloan-Kettering Cancer
Center
• Atara Biotherapeutics
2. MSLN anti-MSLN CAR-T cells secreting PD-1 nanobodies I NCT04489862 r • Wuhan Union Hospital, China
PD-1 • Shanghai Cell Therapy GroupCo.,
Ltd
3. MSLN anti-MSLN CAR-T cell (intraperitoneal MCY-M11 - non-viral, mRNA-based) I NCT03608618* • MaxCyte, Inc.
• CTI Clinical Trial and Consulting
Services
4. MSLN anti-MSLN CAR-T cells (single retroviral vector) I/II NCT01583686* • National Cancer Institute (NCI)
• National Institutes of Health
Clinical Center (CC)
c
5. MSLN anti- MSLN CAR-T cells (lentiviral transduced anti-MSLN immunoreceptor SS1 fused to the 4-1BB and I NCT02159716 • University of Pennsylvania
CD3ζ signaling domains)
c
6. FAP anti-FAP CAR-T cells (retroviral vector) I NCT01722149 • University of Zurich
r
7. MSLN anti-MSLN CAR-T cells (local delivery, lentiviral transduced) I NCT03054298 • University of Pennsylvania
• National Institutes of Health (NIH)
• Tmunity Therapeutics
a,nr
8. MSLN anti-MSLN CAR-T cells (iCasp9M28z CAR-transduced) I/II NCT02414269 • Memorial Sloan-Kettering Cancer
Center
• Bellicum Pharmaceuticals
• United States Department of
Defence
un
9. MSLN anti-MSLN CAR-T cells I NCT02580747 • Chinese PLA General Hospital
10. MSLN anti-MSLN CAR-T cells (mRNA-based, 4-1BB and CD3ζ signaling domains) I NCT01355965 c • University of Pennsylvania
r
11. MSLN, CD19, CD22, CD33, CD38, anti-MSLN, anti-CD19; anti-CD22; anti-CD33 ; anti-CD38; anti-NY-ESO-1; anti-DR5; anti-C-met; anti- I/II NCT03638206 • Shenzhen BinDeBio Ltd.
NY-ESO-1, DR5, C-Met, EGFR V III, EGFR V III; (multi-target gene-modified immunotherapy, CAR-T/TCR-T cells include ten different • The First Affiliated Hospital of
tumour-specific antibodies) Zhengzhou University
Note: These are clinical studies listed on https://clinicaltrials.gov/ [Last accessed on 15 April 2022]. r: Recruiting; c: completed; a: active; nr: not recruiting; un: unknown; *study terminated.
subclasses, which correlates strongly with molecular markers of the epithelial-mesenchymal transition . The interpatient heterogeneity of MM has hampered
[50]
clinical trial design and results. Comprehensive genomic and transcriptomic analysis of MM has revealed that “one-size-fits-all” approaches to therapy are
unsuccessful due to extensive genomic heterogeneity among MM patients. Inactivated tumor suppressor genes, including BAP1, NF2, CDKN2A, and SETD2,
[51]
dominate the mutational landscape in MM . Intratumoral heterogeneity, meaning the presence of distinct tumor cell populations within the same tumor, is