Page 60 - Read Online
P. 60
Diab et al. J Cancer Metastasis Treat 2022;8:42 https://dx.doi.org/10.20517/2394-4722.2022.60 Page 3 of 14
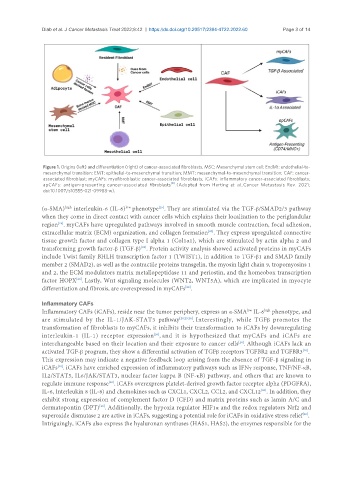
Figure 1. Origins (left) and differentiation (right) of cancer-associated fibroblasts. MSC: Mesenchymal stem cell; EndMt: endothelial-to-
mesenchymal transition; EMT: epithelial-to-mesenchymal transition; MMT: mesenchymal-to-mesenchymal transition; CAF: cancer-
associated fibroblast; myCAFs: myofibroblastic cancer-associated fibroblasts; iCAFs: inflammatory cancer-associated fibroblasts;
[8]
apCAFs: antigen-presenting cancer-associated fibroblasts . (Adopted from Herting et al., Cancer Metastasis Rev. 2021;
doi:10.1007/s10555-021-09988-w).
low
(α-SMA) interleukin-6 (IL-6) phenotype . They are stimulated via the TGF-β/SMAD2/3 pathway
[25]
high
when they come in direct contact with cancer cells which explains their localization to the periglandular
region . myCAFs have upregulated pathways involved in smooth muscle contraction, focal adhesion,
[25]
extracellular matrix (ECM) organization, and collagen formation . They express upregulated connective
[20]
tissue growth factor and collagen type I alpha 1 (Col1α1), which are stimulated by actin alpha 2 and
[25]
transforming growth factor-β (TGF-β) . Protein activity analysis showed activated proteins in myCAFs
include Twist family BHLH transcription factor 1 (TWIST1), in addition to TGF-β1 and SMAD family
member 2 (SMAD2), as well as the contractile proteins transgelin, the myosin light chain 9, tropomyosins 1
and 2, the ECM modulators matrix metallopeptidase 11 and periostin, and the homeobox transcription
factor HOPX . Lastly, Wnt signaling molecules (WNT2, WNT5A), which are implicated in myocyte
[20]
differentiation and fibrosis, are overexpressed in myCAFs .
[20]
Inflammatory CAFs
Inflammatory CAFs (iCAFs), reside near the tumor periphery, express an α-SMA IL-6 phenotype, and
low
high
are stimulated by the IL-1/JAK-STAT3 pathway [20,25,26] . Interestingly, while TGFβ promotes the
transformation of fibroblasts to myCAFs, it inhibits their transformation to iCAFs by downregulating
interleukin-1 (IL-1) receptor expression , and it is hypothesized that myCAFs and iCAFs are
[26]
interchangeable based on their location and their exposure to cancer cells . Although iCAFs lack an
[26]
activated TGF-β program, they show a differential activation of TGFβ receptors TGFBR2 and TGFBR3 .
[20]
This expression may indicate a negative feedback loop arising from the absence of TGF-β signaling in
iCAFs . iCAFs have enriched expression of inflammatory pathways such as IFNγ response, TNF/NF-κB,
[20]
IL2/STAT5, IL6/JAK/STAT3, nuclear factor kappa B (NF-κB) pathway, and others that are known to
regulate immune response . iCAFs overexpress platelet-derived growth factor receptor alpha (PDGFRA),
[20]
IL-6, Interleukin 8 (IL-8) and chemokines such as CXCL1, CXCL2, CCL2, and CXCL12 . In addition, they
[20]
exhibit strong expression of complement factor D (CFD) and matrix proteins such as lamin A/C and
dermatopontin (DPT) . Additionally, the hypoxia regulator HIF1α and the redox regulators Nrf2 and
[20]
[20]
superoxide dismutase 2 are active in iCAFs, suggesting a potential role for iCAFs in oxidative stress relief .
Intriguingly, iCAFs also express the hyaluronan synthases (HAS1, HAS2), the enzymes responsible for the