Page 67 - Read Online
P. 67
Maurizi et al. J Cancer Metastasis Treat 2021;7:35 https://dx.doi.org/10.20517/2394-4722.2021.74 Page 5 of 19
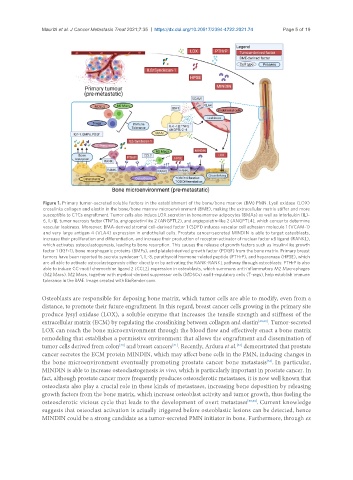
Figure 1. Primary tumor-secreted soluble factors in the establishment of the bone/bone marrow (BM) PMN. Lysil oxidase (LOX)
crosslinks collagen and elastin in the bone/bone marrow microenvironment (BME), making the extracellular matrix stiffer and more
susceptible to CTCs engraftment. Tumor cells also induce LOX secretion in bone marrow adipocytes (BMAs) as well as Interleukin (IL)-
6, IL-1β, tumor necrosis factor (TNF)α, angiopoietin-like 2 (ANGPTL2), and angiopoietin-like 2 (ANGPTL4), which concur to determine
vascular leakiness. Moreover, BMA-derived stromal cell-derived factor 1 (SDF1) induces vascular cell adhesion molecule 1 (VCAM-1)
and very large antigen 4 (VLA4) expression in endothelial cells. Prostate cancer-secreted MINDIN is able to target osteoblasts,
increase their proliferation and differentiation, and increase their production of receptor-activator of nuclear factor κB ligand (RANKL),
which activates osteoclastogenesis, leading to bone resorption. This causes the release of growth factors such as insulin-like growth
factor 1 (IGF-1), bone morphogenic proteins (BMPs), and platelet-derived growth factor (PDGF) from the bone matrix. Primary breast
tumors have been reported to secrete syndecan-1, IL-8, parathyroid hormone-related peptide (PTHrP), and heparanase (HPSE), which
are all able to activate osteoclastogenesis either directly or by activating the RANK-RANKL pathway through osteoblasts. PTHrP is also
able to induce CC-motif chemochine ligand 2 (CCL2) expression in osteoblasts, which summons anti-inflammatory M2 Macrophages
(M2 Macs). M2 Macs, together with myeloid-derived suppressor cells (MDSCs) and t-regulatory cells (T-regs), help establish immune
tolerance in the BME. Image created with BioRender.com.
Osteoblasts are responsible for deposing bone matrix, which tumor cells are able to modify, even from a
distance, to promote their future engraftment. In this regard, breast cancer cells growing in the primary site
produce lysyl oxidase (LOX), a soluble enzyme that increases the tensile strength and stiffness of the
extracellular matrix (ECM) by regulating the crosslinking between collagen and elastin [28,29] . Tumor-secreted
LOX can reach the bone microenvironment through the blood flow and effectively enact a bone matrix
remodeling that establishes a permissive environment that allows the engraftment and dissemination of
tumor cells derived from colon and breast cancers . Recently, Ardura et al. demonstrated that prostate
[30]
[32]
[31]
cancer secretes the ECM protein MINDIN, which may affect bone cells in the PMN, inducing changes in
the bone microenvironment eventually promoting prostate cancer bone metastasis . In particular,
[32]
MINDIN is able to increase osteoclastogenesis in vivo, which is particularly important in prostate cancer. In
fact, although prostate cancer more frequently produces osteosclerotic metastases, it is now well known that
osteoclasts also play a crucial role in these kinds of metastases, increasing bone deposition by releasing
growth factors from the bone matrix, which increase osteoblast activity and tumor growth, thus fueling the
osteosclerotic vicious cycle that leads to the development of overt metastases [33,34] . Current knowledge
suggests that osteoclast activation is actually triggered before osteoblastic lesions can be detected, hence
MINDIN could be a strong candidate as a tumor-secreted PMN initiator in bone. Furthermore, through ex