Page 14 - Read Online
P. 14
Abughanimeh et al. J Cancer Metastasis Treat 2020;6:50 I http://dx.doi.org/10.20517/2394-4722.2020.110 Page 9 of 13
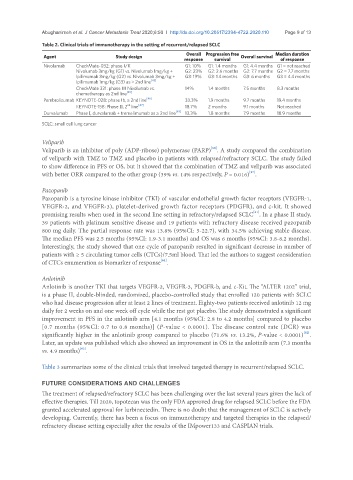
Table 2. Clinical trials of immunotherapy in the setting of recurrent/relapsed SCLC
Agent Study design Overall Progression free Overall survival Median duration
response survival of response
Nivolumab CheckMate-032: phase I/II G1: 10% G1: 1.4 months G1: 4.4 months G1 = not reached
Nivolumab 3mg/kg (G1) vs. Nivolumab 1mg/kg + G2: 23% G2: 2.6 months G2: 7.7 months G2 = 7.7 months
Ipilimumab 3mg/kg (G2) vs. Nivolumab 3mg/kg + G3: 19% G3: 1.4 months G3: 6 months G3 = 4.4 months
Ipilimumab 1mg/kg (G3) as ≥ 2nd line [43]
CheckMate 331: phase III Nivolumab vs. 14% 1.4 months 7.5 months 8.3 months
chemotherapy as 2nd line [45]
Pembrolizumab KEYNOTE-028: phase Ib, ≥ 2nd line [46] 33.3% 1.9 months 9.7 months 19.4 months
nd
KEYNOTE-158: Phase II, 2 line [47] 18.7% 2 months 9.1 months Not reached
Durvalumab Phase I, durvalumab + tremelimumab as ≥ 2nd line [49] 13.3% 1.8 months 7.9 months 18.9 months
SCLC: small cell lung cancer
Veliparib
Veliparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) . A study compared the combination
[60]
of veliparib with TMZ to TMZ and placebo in patients with relapsed/refractory SCLC. The study failed
to show difference in PFS or OS, but it showed that the combination of TMZ and veliparib was associated
[27]
with better ORR compared to the other group (39% vs. 14% respectively, P = 0.016) .
Pazopanib
Pazopanib is a tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors (VEGFR-1,
VEGFR-2, and VEGFR-3), platelet-derived growth factor receptors (PDGFR), and c-kit. It showed
[61]
promising results when used in the second line setting in refractory/relapsed SCLC . In a phase II study,
39 patients with platinum sensitive disease and 19 patients with refractory disease received pazopanib
800 mg daily. The partial response rate was 13.8% (95%CI: 5-22.7), with 34.5% achieving stable disease.
The median PFS was 2.5 months (95%CI: 1.9-3.1 months) and OS was 6 months (95%CI: 3.8-8.2 months).
Interestingly, the study showed that one cycle of pazopanib resulted in significant decrease in number of
patients with ≥ 5 circulating tumor cells (CTCs)/7.5ml blood. That led the authors to suggest consideration
of CTCs enumeration as biomarker of response .
[61]
Anlotinib
Anlotinib is another TKI that targets VEGFR-2, VEGFR-3, PDGFR-b, and c-Kit. The “ALTER 1202” trial,
is a phase II, double-blinded, randomized, placebo-controlled study that enrolled 120 patients with SCLC
who had disease progression after at least 2 lines of treatment. Eighty-two patients received anlotinib 12 mg
daily for 2 weeks on and one week off cycle while the rest got placebo. The study demonstrated a significant
improvement in PFS in the anlotinib arm [4.1 months (95%CI: 2.8 to 4.2 months] compared to placebo
[0.7 months (95%CI: 0.7 to 0.8 months)] (P-value < 0.0001). The disease control rate (DCR) was
[62]
significantly higher in the anlotinib group compared to placebo (71.6% vs. 13.2%, P-value < 0.0001) .
Later, an update was published which also showed an improvement in OS in the anlotinib arm (7.3 months
[63]
vs. 4.9 months) .
Table 3 summarizes some of the clinical trials that involved targeted therapy in recurrent/relapsed SCLC.
FUTURE CONSIDERATIONS AND CHALLENGES
The treatment of relapsed/refractory SCLC has been challenging over the last several years given the lack of
effective therapies. Till 2020, topotecan was the only FDA approved drug for relapsed SCLC before the FDA
granted accelerated approval for lurbinectedin. There is no doubt that the management of SCLC is actively
developing. Currently, there has been a focus on immunotherapy and targeted therapies in the relapsed/
refractory disease setting especially after the results of the IMpower133 and CASPIAN trials.