Page 88 - Read Online
P. 88
D’Amico et al. J Cancer Metastasis Treat 2021;7:3 I http://dx.doi.org/10.20517/2394-4722.2020.93 Page 3 of 16
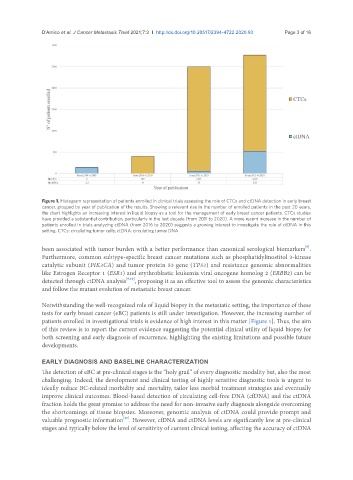
Figure 1. Histogram representation of patients enrolled in clinical trials assessing the role of CTCs and ctDNA detection in early breast
cancer, grouped by year of publication of the results. Showing a relevant rise in the number of enrolled patients in the past 20 years,
the chart highlights an increasing interest in liquid biopsy as a tool for the management of early breast cancer patients. CTCs studies
have provided a substantial contribution, particularly in the last decade (from 2011 to 2020). A more recent increase in the number of
patients enrolled in trials analyzing ctDNA (from 2016 to 2020) suggests a growing interest to investigate the role of ctDNA in this
setting. CTCs: circulating tumor cells; ctDNA: circulating tumor DNA
[8]
been associated with tumor burden with a better performance than canonical serological biomarkers .
Furthermore, common subtype-specific breast cancer mutations such as phosphatidylinostitol 3-kinase
catalytic subunit (PIK3CA) and tumor protein 53 gene (TP53) and resistance genomic abnormalities
like Estrogen Receptor 1 (ESR1) and erythroblastic leukemia viral oncogene homolog 2 (ERBB2) can be
detected through ctDNA analysis [9,10] , proposing it as an effective tool to assess the genomic characteristics
and follow the mutant evolution of metastatic breast cancer.
Notwithstanding the well-recognized role of liquid biopsy in the metastatic setting, the importance of these
tests for early breast cancer (eBC) patients is still under investigation. However, the increasing number of
patients enrolled in investigational trials is evidence of high interest in this matter [Figure 1]. Thus, the aim
of this review is to report the current evidence suggesting the potential clinical utility of liquid biopsy for
both screening and early diagnosis of recurrence, highlighting the existing limitations and possible future
developments.
EARLY DIAGNOSIS AND BASELINE CHARACTERIZATION
The detection of eBC at pre-clinical stages is the “holy grail” of every diagnostic modality but, also the most
challenging. Indeed, the development and clinical testing of highly sensitive diagnostic tools is urgent to
ideally reduce BC-related morbidity and mortality, tailor less morbid treatment strategies and eventually
improve clinical outcomes. Blood-based detection of circulating cell-free DNA (cfDNA) and the ctDNA
fraction holds the great promise to address the need for non-invasive early diagnosis alongside overcoming
the shortcomings of tissue biopsies. Moreover, genomic analysis of ctDNA could provide prompt and
[11]
valuable prognostic information . However, cfDNA and ctDNA levels are significantly low at pre-clinical
stages and typically below the level of sensitivity of current clinical testing, affecting the accuracy of ctDNA