Page 109 - Read Online
P. 109
Gawel et al. J Cancer Metastasis Treat 2022;8:26 https://dx.doi.org/10.20517/2394-4722.2022.13 Page 5 of 11
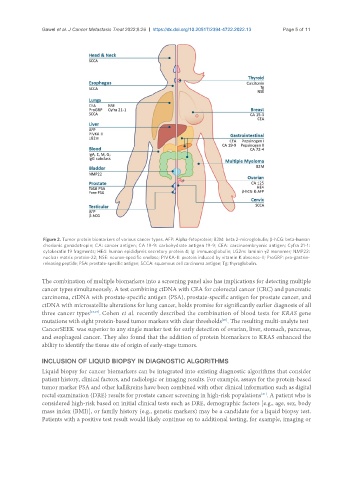
Figure 2. Tumor protein biomarkers of various cancer types. AFP: Alpha-fetoprotein; B2M: beta 2-microglobulin; β-hCG: beta-human
chorionic gonadotropin; CA: cancer antigen; CA 19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; Cyfra 21-1:
cytokeratin 19 fragments; HE4: human epididymis secretory protein 4; Ig: immunoglobulin; LG2m: laminin γ2 monomer; NMP22:
nuclear matrix protein-22; NSE: neuron-specific enolase; PIVKA-II: protein induced by vitamin K absence-II; ProGRP: pro-gastrin-
releasing peptide; PSA: prostate-specific antigen; SCCA: squamous cell carcinoma antigen; Tg: thyroglobulin.
The combination of multiple biomarkers into a screening panel also has implications for detecting multiple
cancer types simultaneously. A test combining ctDNA with CEA for colorectal cancer (CRC) and pancreatic
carcinoma, ctDNA with prostate-specific antigen (PSA), prostate-specific antigen for prostate cancer, and
ctDNA with microsatellite alterations for lung cancer, holds promise for significantly earlier diagnosis of all
three cancer types [18,19] . Cohen et al. recently described the combination of blood tests for KRAS gene
[20]
mutations with eight protein-based tumor markers with clear thresholds . The resulting multi-analyte test–
CancerSEEK–was superior to any single marker test for early detection of ovarian, liver, stomach, pancreas,
and esophageal cancer. They also found that the addition of protein biomarkers to KRAS enhanced the
ability to identify the tissue site of origin of early-stage tumors.
INCLUSION OF LIQUID BIOPSY IN DIAGNOSTIC ALGORITHMS
Liquid biopsy for cancer biomarkers can be integrated into existing diagnostic algorithms that consider
patient history, clinical factors, and radiologic or imaging results. For example, assays for the protein-based
tumor marker PSA and other kallikreins have been combined with other clinical information such as digital
[21]
rectal examination (DRE) results for prostate cancer screening in high-risk populations . A patient who is
considered high-risk based on initial clinical tests such as DRE, demographic factors [e.g., age, sex, body
mass index (BMI)], or family history (e.g., genetic markers) may be a candidate for a liquid biopsy test.
Patients with a positive test result would likely continue on to additional testing, for example, imaging or