Page 222 - Read Online
P. 222
Page 14 of 20 dos Santos et al. J Cancer Metastasis Treat 2019;5:25 I http://dx.doi.org/10.20517/2394-4722.2018.83
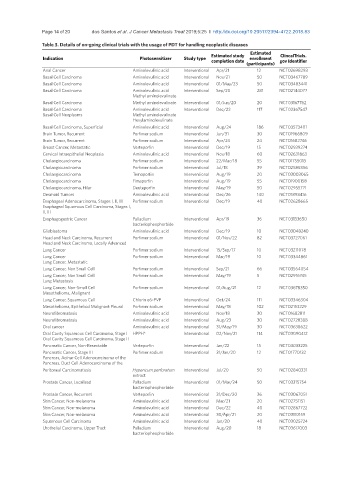
Table 3. Details of on-going clinical trials with the usage of PDT for handling neoplastic diseases
Estimated
Estimated study ClincalTrials.
Indication Photosensitizer Study type enrollment
completion date gov Identifier
(participants)
Anal Cancer Aminolevulinic acid Interventional Apr/21 12 NCT02698293
Basal Cell Carcinoma Aminolevulinic acid Interventional Nov/21 50 NCT03467789
Basal Cell Carcinoma Aminolevulinic acid Interventional 01/May/23 50 NCT03483441
Basal Cell Carcinoma Aminolevulinic acid Interventional Sep/20 281 NCT02144077
Methyl aminolevulinate
Basal Cell Carcinoma Methyl aminolevulinate Interventional 01/Jun/20 20 NCT03167762
Basal Cell Carcinoma Aminolevulinic acid Interventional Dec/22 117 NCT02367547
Basal Cell Neoplasms Methyl aminolevulinate
Hexylaminolevulinate
Basal Cell Carcinoma, Superficial Aminolevulinic acid Interventional Aug/24 186 NCT03573401
Brain Tumor, Recurrent Porfimer sodium Interventional Jun/31 30 NCT01966809
Brain Tumor, Recurrent Porfimer sodium Interventional Apr/24 24 NCT01682746
Breast Cancer, Metastatic Verteporfin Interventional Dec/19 15 NCT02939274
Cervical Intraepithelial Neoplasia Aminolevulinic acid Interventional Nov/18 60 NCT02631863
Cholangiocarcinoma Porfimer sodium Interventional 22/Mar/18 55 NCT01755013
Cholangiocarcinoma Porfimer sodium Interventional Jul/18 39 NCT02585856
Cholangiocarcinoma Temoporfin Interventional Aug/19 20 NCT03003065
Cholangiocarcinoma Fimaporfin Interventional Aug/19 55 NCT01900158
Cholangiocarcinoma, Hilar Deuteporfin Interventional May/19 50 NCT02955771
Desmoid Tumors Aminolevulinic acid Interventional Dec/26 140 NCT01898416
Esophageal Adenocarcinoma, Stages I, II, III Porfimer sodium Interventional Dec/19 40 NCT02628665
Esophageal Squamous Cell Carcinoma, Stages I,
II, III
Esophagogastric Cancer Palladium Interventional Apr/19 36 NCT03133650
bacteriopheophorbide
Glioblastoma Aminolevulinic acid Interventional Dec/19 10 NCT03048240
Head and Neck Carcinoma, Recurrent Porfimer sodium Interventional 01/Nov/22 82 NCT03727061
Head and Neck Carcinoma, Locally Advanced
Lung Cancer Porfimer sodium Interventional 15/Sep/17 10 NCT03211078
Lung Cancer Porfimer sodium Interventional Mar/19 10 NCT03344861
Lung Cancer, Metastatic
Lung Cancer, Non Small Cell Porfimer sodium Interventional Sep/21 66 NCT03564054
Lung Cancer, Non Small Cell Porfimer sodium Interventional May/19 5 NCT02916745
Lung Metastasis
Lung Cancer, Non-Small Cell Porfimer sodium Interventional 01/Aug/21 12 NCT03678350
Mesothelioma, Malignant
Lung Cancer, Squamous Cell Chlorin e6-PVP Interventional Oct/24 111 NCT03346304
Mesothelioma, Epitheliod Malignant Pleural Porfimer sodium Interventional May/18 102 NCT02153229
Neurofibromatosis Aminolevulinic acid Interventional Nov/18 30 NCT01682811
Neurofibromatosis Aminolevulinic acid Interventional Aug/23 30 NCT02728388
Oral cancer Aminolevulinic acid Interventional 31/May/19 30 NCT03638622
Oral Cavity Squamous Cell Carcinoma, Stage I HPPH* Interventional 02/Nov/21 114 NCT03090412
Oral Cavity Squamous Cell Carcinoma, Stage II
Pancreatic Cancer, Non-Resectable Verteporfin Interventional Jan/22 15 NCT03033225
Pancreatic Cancer, Stage III Porfimer sodium Interventional 31/Jan/20 12 NCT01770132
Pancreas, Acinar Cell Adenocarcinoma of the
Pancreas, Duct Cell Adenocarcinoma of the
Peritoneal Carcinomatosis Hypericum perforatum Interventional Jul/20 50 NCT02840331
extract
Prostate Cancer, Localized Palladium Interventional 01/Mar/24 50 NCT03315754
bacteriopheophorbide
Prostate Cancer, Recurrent Verteporfin Interventional 31/Dec/20 36 NCT03067051
Skin Cancer, Non-melanoma Aminolevulinic acid Interventional Mar/21 20 NCT02751151
Skin Cancer, Non-melanoma Aminolevulinic acid Interventional Dec/22 40 NCT02867722
Skin Cancer, Non-melanoma Aminolevulinic acid Interventional 30/Apr/21 20 NCT03110159
Squamous Cell Carcinoma Aminolevulinic acid Interventional Jan/20 40 NCT03025724
Urothelial Carcinoma, Upper Tract Palladium Interventional Aug/20 18 NCT03617003
bacteriopheophorbide