Page 13 - Read Online
P. 13
Page 4 of 14 Di Raimo et al. J Cancer Metastasis Treat 2018;4:54 I http://dx.doi.org/10.20517/2394-4722.2018.50
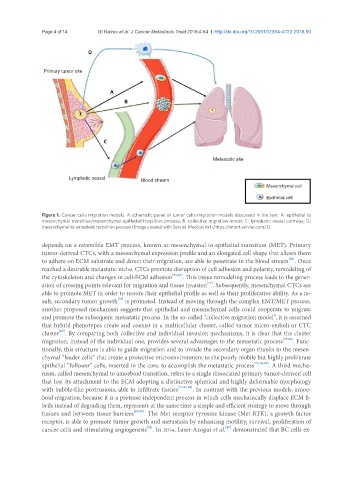
Primary tumor site
Metastatic site
Lymphatic vessel Blood stream
Mesenchymal cell
Epithelial cell
Figure 1. Cancer cells migration models. A schematic panel of tumor cells migration models discussed in the text: A: epithelial to
mesenchymal transition/mesenchymal-epithelial transition process; B: collective migration model; C: lymphatic vessel pathway; D:
mesenchymal to amoeboid transition process [Image created with Servier Medical Art (https://smart.servier.com/)]
depends on a reversible EMT process, known as mesenchymal to epithelial transition (MET). Primary
tumor-derived CTCs, with a mesenchymal expression profile and an elongated cell shape that allows them
[82]
to adhere on ECM substrate and direct their migration, are able to penetrate in the blood stream . Once
reached a desirable metastatic niche, CTCs promote disruption of cell adhesion and polarity, remodeling of
the cytoskeleton and changes in cell-ECM adhesion [83,84] . This tissue remodeling process leads to the gener-
[77]
ation of crossing points relevant for migration and tissue invasion . Subsequently, mesenchymal CTCs are
able to promote MET in order to restore their epithelial profile as well as their proliferative ability. As a re-
sult, secondary tumor growth is promoted. Instead of moving through the complex EMT/MET process,
[78]
another proposed mechanism suggests that epithelial and mesenchymal cells could cooperate to migrate
and promote the subsequent metastatic process. In the so-called “collective migration model”, it is assumed
that hybrid phenotypes create and coexist in a multicellular cluster, called tumor micro-emboli or CTC
[85]
cluster . By comparing both collective and individual invasion mechanisms, it is clear that the cluster
migration, instead of the individual one, provides several advantages to the metastatic process [77,82] . Func-
tionally, this structure is able to guide migration and to invade the secondary organ thanks to the mesen-
chymal “leader cells” that create a protective microenvironment to the poorly mobile but highly proliferate
epithelial “follower” cells, inserted in the core, to accomplish the metastatic process [76,78,86] . A third mecha-
nism, called mesenchymal to amoeboid transition, refers to a single dissociated primary tumor-derived cell
that lost its attachment to the ECM adopting a distinctive spherical and highly deformable morphology
with bubble-like protrusions, able to infiltrate tissues [77,87,88] . In contrast with the previous models, amoe-
boid migration, because it is a protease-independent process in which cells mechanically displace ECM fi-
brils instead of degrading them, represents at the same time a simple and efficient strategy to move through
tissues and between tissue barriers [89,90] . The Met receptor tyrosine kinase (Met-RTK), a growth factor
receptor, is able to promote tumor growth and metastasis by enhancing motility, survival, proliferation of
cancer cells and stimulating angiogenesis . In 2014, Laser-Azogui et al. demonstrated that BC cells ex-
[91]
[87]