Page 53 - Read Online
P. 53
Tanasanvimon. J Cancer Metastasis Treat 2018;4:57 I http://dx.doi.org/10.20517/2394-4722.2018.38 Page 7 of 11
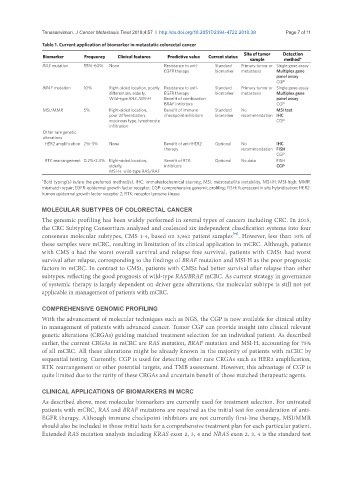
Table 1. Current application of biomarker in metastatic colorectal cancer
Biomarker Frequency Clinical features Predictive value Current status Site of tumor Detection
sample method*
RAS mutation 55%-60% None Resistance to anti- Standard Primary tumor or Single gene assay
EGFR therapy biomarker metastasis Multiplex gene
panel assay
CGP
BRAF mutation 10% Right-sided location, poorly Resistance to anti- Standard Primary tumor or Single gene assay
differention, elderly, EGFR therapy biomarker metastasis Multiplex gene
Wild-type RAS, MSI-H Benefit of combination panel assay
BRAF inhibitors CGP
MSI/MMR 5% Right-sided location, Benefit of immune Standard No MSI test
poor differentiation, checkpoint inhibitors biomarker recommendation IHC
mucinous type, lymphocyte CGP
infiltration
Other rare genetic
alterations
HER2 amplification 2%-3% None Benefit of anti-HER2 Optional No IHC
therapy recommendation FISH
CGP
RTK rearrangement 0.2%-2.4% Right-sided location, Benefit of RTK Optional No data FISH
elderly, inhibitors CGP
MSI-H, wild-type RAS/RAF
*Bold typing(s) is/are the preferred method(s). IHC: immuhistochemical staining; MSI: microsatellite instability; MSI-H: MSI-high; MMR:
mismatch repair; EGFR: epidermal growth factor receptor; CGP: comprehensive genomic profiling; FISH: fluorescent in situ hybridization; HER2:
human epidermal growth factor receptor 2; RTK: receptor tyrosine kinase
MOLECULAR SUBTYPES OF COLORECTAL CANCER
The genomic profiling has been widely performed in several types of cancers including CRC. In 2015,
the CRC Subtyping Consortium analyzed and coalesced six independent classification systems into four
[91]
consensus molecular subtypes, CMS 1-4, based on 3,962 patient samples . However, less than 10% of
these samples were mCRC, resulting in limitation of its clinical application in mCRC. Although, patients
with CMS 4 had the worst overall survival and relapse free survival, patients with CMS1 had worst
survival after relapse, corresponding to the findings of BRAF mutation and MSI-H as the poor prognostic
factors in mCRC. In contrast to CMS1, patients with CMS2 had better survival after relapse than other
subtypes, reflecting the good prognosis of wild-type RAS/BRAF mCRC. As current strategy in governance
of systemic therapy is largely dependent on driver gene alterations, the molecular subtype is still not yet
applicable in management of patients with mCRC.
COMPREHENSIVE GENOMIC PROFILING
With the advancement of molecular techniques such as NGS, the CGP is now available for clinical utility
in management of patients with advanced cancer. Tumor CGP can provide insight into clinical relevant
genetic alterations (CRGAs) guiding matched treatment selection for an individual patient. As described
earlier, the current CRGAs in mCRC are RAS mutation, BRAF mutation and MSI-H, accounting for 75%
of all mCRC. All these alterations might be already known in the majority of patients with mCRC by
sequential testing. Currently, CGP is used for detecting other rare CRGAs such as HER2 amplification,
RTK rearrangement or other potential targets, and TMB assessment. However, this advantage of CGP is
quite limited due to the rarity of these CRGAs and uncertain benefit of those matched therapeutic agents.
CLINICAL APPLICATIONS OF BIOMARKERS IN MCRC
As described above, most molecular biomarkers are currently used for treatment selection. For untreated
patients with mCRC, RAS and BRAF mutations are required as the initial test for consideration of anti-
EGFR therapy. Although immune checkpoint inhibitors are not currently first-line therapy, MSI/MMR
should also be included in those initial tests for a comprehensive treatment plan for each particular patient.
Extended RAS mutation analysis including KRAS exon 2, 3, 4 and NRAS exon 2, 3, 4 is the standard test