Page 38 - Read Online
P. 38
Page 6 of 14 Bittoni et al. J Cancer Metastasis Treat 2018;4:55 I http://dx.doi.org/10.20517/2394-4722.2018.37
T cell
(1) Tumor cell
PD-L1
Tumor cell
PD-L1
T cell
APC CD 80 (3)
CTLA-4 Tumor cell PD-L2
CD 86
CD 28 PD-L1
T cell (2) PD-L1
MHC
TCR Macrophage Macrophage
(1) Naive T cell; (2) Primed against antigen presented by APC; (3) Inactivated by PD-L1/L2
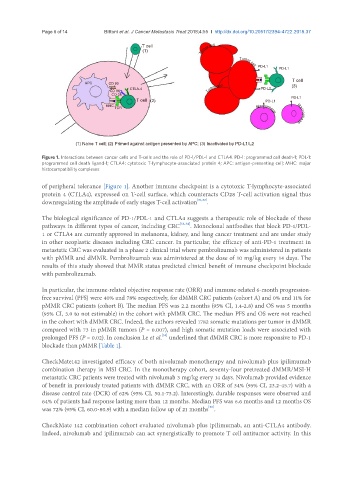
Figure 1. Interactions between cancer cells and T-cells and the role of PD-1/PDL-1 and CTLA4. PD-1: programmed cell death-1; PDL-1:
programmed cell death ligand-1; CTLA4: cytotoxic T-lymphocyte-associated protein 4; APC: antigen-presenting cell; MHC: major
histocompatibility complexes
of peripheral tolerance [Figure 1]. Another immune checkpoint is a cytotoxic T-lymphocyte-associated
protein 4 (CTLA4), expressed on T-cell surface, which counteracts CD28 T-cell activation signal thus
downregulating the amplitude of early stages T-cell activation [31,32] .
The biological significance of PD-1/PDL-1 and CTLA4 suggests a therapeutic role of blockade of these
pathways in different types of cancer, including CRC [33,34] . Monoclonal antibodies that block PD-1/PDL-
1 or CTLA4 are currently approved in melanoma, kidney, and lung cancer treatment and are under study
in other neoplastic diseases including CRC cancer. In particular, the efficacy of anti-PD-1 treatment in
metastatic CRC was evaluated in a phase 2 clinical trial where pembrolizumab was administered in patients
with pMMR and dMMR. Pembrolizumab was administered at the dose of 10 mg/kg every 14 days. The
results of this study showed that MMR status predicted clinical benefit of immune checkpoint blockade
with pembrolizumab.
In particular, the immune-related objective response rate (ORR) and immune-related 6-month progression-
free survival (PFS) were 40% and 78% respectively, for dMMR CRC patients (cohort A) and 0% and 11% for
pMMR CRC patients (cohort B). The median PFS was 2.2 months (95% CI, 1.4-2.8) and OS was 5 months
(95% CI, 3.0 to not estimable) in the cohort with pMMR CRC. The median PFS and OS were not reached
in the cohort with dMMR CRC. Indeed, the authors revealed 1782 somatic mutations per tumor in dMMR
compared with 73 in pMMR tumors (P = 0.007), and high somatic mutation loads were associated with
[35]
prolonged PFS (P = 0.02). In conclusion Le et al. underlined that dMMR CRC is more responsive to PD-1
blockade than pMMR [Table 1].
CheckMate142 investigated efficacy of both nivolumab monotherapy and nivolumab plus ipilimumab
combination therapy in MSI CRC. In the monotherapy cohort, seventy-four pretreated dMMR/MSI-H
metastatic CRC patients were treated with nivolumab 3 mg/kg every 14 days. Nivolumab provided evidence
of benefit in previously treated patients with dMMR CRC, with an ORR of 34% (95% CI, 23.2-45.7) with a
disease control rate (DCR) of 62% (95% CI, 50.1-73.2). Interestingly, durable responses were observed and
64% of patients had response lasting more than 12 months. Median PFS was 6.6 months and 12 months OS
[36]
was 72% (95% CI, 60.0-80.9) with a median follow up of 21 months .
CheckMate 142 combination cohort evaluated nivolumab plus ipilimumab, an anti-CTLA4 antibody.
Indeed, nivolumab and ipilimumab can act synergistically to promote T cell antitumor activity. In this