Page 97 - Read Online
P. 97
Toihata et al. J Cancer Metastasis Treat 2018;4:24 I http://dx.doi.org/10.20517/2394-4722.2017.82 Page 3 of 10
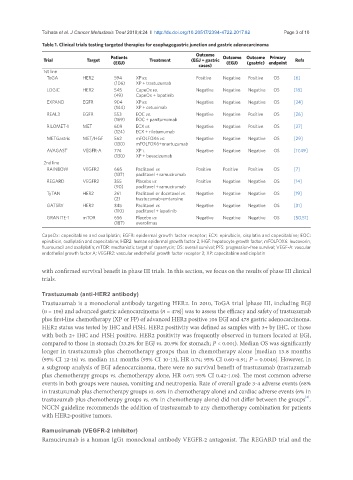
Table 1. Clinical trials testing targeted therapies for esophagogastric junction and gastric adenocarcinoma
Outcome
Patients Outcome Outcome Primary
Trial Target Treatment (EGJ + gastric Refs
(EGJ) (EGJ) (gastric) endpoint
cases)
1st line
ToGA HER2 594 XP vs. Positive Negative Positive OS [6]
(106) XP + trastuzumab
LOGiC HER2 545 CapeOx vs. Negative Negative Negative OS [18]
(49) CapeOx + lapatinib
EXPAND EGFR 904 XP vs. Negative Negative Negative OS [24]
(144) XP + cetuximab
REAL3 EGFR 553 EOC vs. Negative Negative Positive OS [26]
(169) EOC + panitumumab
RILOMET-1 MET 609 ECX vs. Negative Negative Positive OS [27]
(124) ECX + rilotumumab
METGastric MET/HGF 562 mFOLFOX6 vs. Negative Negative Negative OS [29]
(130) mFOLFOX6+onartuzumab
AVAGAST VEGFR-A 774 XP i. Negative Negative Negative OS [17,49]
(130) XP + bevacizumab
2nd line
RAINBOW VEGFR2 665 Paclitaxel vs. Positive Positive Positive OS [7]
(137) paclitaxel + ramucirumab
REGARD VEGFR2 355 Placebo vs. Positive Negative Negative OS [14]
(90) paclitaxel + ramucirumab
TyTAN HER2 261 Paclitaxel or docetaxel vs. Negative Negative Negative OS [19]
(2) trastuzumab-emtansine
GATSBY HER2 345 Paclitaxel vs. Negative Negative Negative OS [31]
(110) paclitaxel + lapatinib
GRANITE-1 mTOR 656 Placebo vs. Negative Negative Negative OS [50,51]
(187) everolimus
CapeOx: capecitabine and oxaliplatin; EGFR: epidermal growth factor receptor; ECX: epirubicin, cisplatin and capecitabine; EOC:
epirubicin, oxaliplatin and capecitabine; HER2: human epidermal growth factor 2; HGF: hepatocyte growth factor; mFOLFOX6: leucovorin,
fluorouracil and oxaliplatin; mTOR: mechanistic target of rapamycin; OS: overall survival; PFS: progression-free survival; VEGF-A: vascular
endothelial growth factor A; VEGFR2: vascular endothelial growth factor receptor 2; XP: capecitabine and cisplatin
with confirmed survival benefit in phase III trials. In this section, we focus on the results of phase III clinical
trials.
Trastuzumab (anti-HER2 antibody)
Trastuzumab is a monoclonal antibody targeting HER2. In 2010, ToGA trial [phase III, including EGJ
(n = 106) and advanced gastric adenocarcinoma (n = 478)] was to assess the efficacy and safety of trastuzumab
plus first-line chemotherapy (XP or FP) of advanced HER2 positive 106 EGJ and 478 gastric adenocarcinoma.
HER2 status was tested by IHC and FISH. HER2 positivity was defined as samples with 3+ by IHC, or those
with both 2+ IHC and FISH positive. HER2 positivity was frequently observed in tumors located at EGJ,
compared to those in stomach (33.2% for EGJ vs. 20.9% for stomach; P < 0.001). Median OS was significantly
longer in trastuzumab plus chemotherapy groups than in chemotherapy alone [median 13.8 months
(95% CI 12-16) vs. median 11.1 months (95% CI 10-13), HR 0.74; 95% CI 0.60-0.91; P = 0.0046]. However, in
a subgroup analysis of EGJ adenocarcinoma, there were no survival benefit of trastuzumab (trastuzumab
plus chemotherapy groups vs. chemotherapy alone, HR 0.67; 95% CI 0.42-1.08). The most common adverse
events in both groups were nausea, vomiting and neutropenia. Rate of overall grade 3-4 adverse events (68%
in trastuzumab plus chemotherapy groups vs. 68% in chemotherapy alone) and cardiac adverse events (6% in
[6]
trastuzumab plus chemotherapy groups vs. 6% in chemotherapy alone) did not differ between the groups .
NCCN guideline recommends the addition of trastuzumab to any chemotherapy combination for patients
with HER2-positive tumors.
Ramucirumab (VEGFR-2 inhibitor)
Ramucirumab is a human IgG1 monoclonal antibody VEGFR-2 antagonist. The REGARD trial and the