Page 79 - Read Online
P. 79
Eto et al. J Cancer Metastasis Treat 2018;4:23 I http://dx.doi.org/10.20517/2394-4722.2017.73 Page 3 of 9
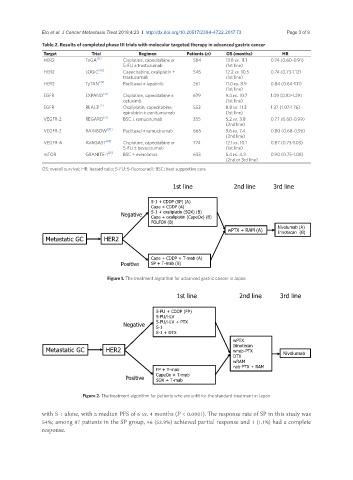
Table 2. Results of completed phase III trials with molecular targeted therapy in advanced gastric cancer
Target Trial Regimen Patients (n) OS (months) HR
HER2 ToGA [21] Cisplatine, capecitabine or 584 13.8 vs. 11.1 0.74 (0.60-0.91)
5-FU ± trastuzumab (1st line)
HER2 LOGiC [48] Capecitabine, oxaliplatin ± 545 12.2 vs. 10.5 0.74 (0.73-1.12)
trastuzumab (1st line)
HER2 TyTAN [49] Paclitaxel ± lapatinib 261 11.0 vs. 8.9 0.84 (0.64-1.11)
(1st line)
EGFR EXPAND [26] Cisplatine, capecitabine ± 679 9.4 vs. 10.7 1.09 (0.92-1.29)
cetuximb (1st line)
EGFR REAL3 [27] Oxaliplatin, capecitabine, 553 8.8 vs. 11.3 1.37 (1.07-1.76)
epirubicin ± panitumumab (1st line)
VEGFR-2 REGARD [31] BSC ± ramucirumab 355 5.2 vs. 3.8 0.77 (0.60-0.99)
(2nd line)
VEGFR-2 RAINBOW [30] Paclitaxel ± ramucirumab 665 9.6 vs. 7.4 0.80 (0.68-0.96)
(2nd line)
VEGFR-A AVAGAST [24] Cisplatine, capecitabine or 774 12.1 vs. 10.1 0.87 (0.73-1.03)
5-FU ± bevacizumab (1st line)
mTOR GRANITE-1 [25] BSC ± everolimus 633 5.4 vs. 4.3 0.90 (0.75-1.08)
(2nd or 3rd line)
OS: overall survival; HR: hazard ratio; 5-FU: 5-fluorouracil; BSC: best supportive care
1st line 2nd line 3rd line
S-1 + CDDP (SP) (A)
Cape + CDDP (A)
Negative S-1 + oxaliplatin (SOX) (B)
Cape + oxaliplatin (CapeOx) (B)
FOLFOX (B)
wPTX + RAM (A) Nivolumab (A)
Irinotecan (B)
Metastatic GC HER2
Cape + CDDP + T-mab (A)
Positive SP + T-mab (B)
Figure 1. The treatment algorithm for advanced gastric cancer in Japan
1st line 2nd line 3rd line
5-FU + CDDP (FP)
5-FU/l-LV
Negative 5-FU/l-LV + PTX
S-1
S-1 + DTX
wPTX
Irinotecan
Metastatic GC HER2 wnab-PTX Nivolumab
DTX
wRAM
nab-PTX + RAM
FP + T-mab
CapeOx + T-mab
Positive SOX + T-mab
Figure 2. The treatment algorithm for patients who are unfit for the standard treatment in Japan
with S-1 alone, with a median PFS of 6 vs. 4 months (P < 0.0001). The response rate of SP in this study was
54%; among 87 patients in the SP group, 46 (52.9%) achieved partial response and 1 (1.1%) had a complete
response.