Page 31 - Read Online
P. 31
Page 4 of 16 Sugarbaker. J Cancer Metastasis Treat 2018;4:7 I http://dx.doi.org/10.20517/2394-4722.2017.67
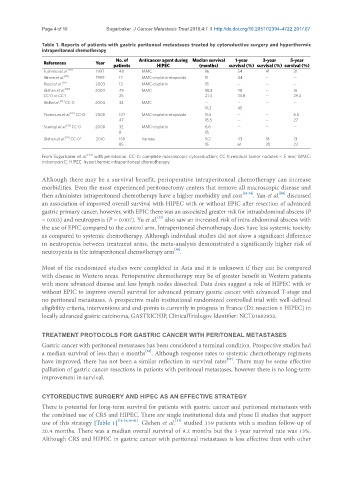
Table 1. Reports of patients with gastric peritoneal metastases treated by cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy
No. of Anticancer agent during Median survival 1-year 3-year 5-year
References Year patients HIPEC (months) survival (%) survival (%) survival (%)
Fujimoto et al. [20] 1997 48 MMC 16 54 41 31
Hirose et al. [38] 1999 17 MMC-cisplatin-etoposide 11 44 -- --
Rossi et al. [39] 2003 13 MMC-cisplatin 15 -- -- --
Glehen et al. [40] 2004 49 MMC 10.3 48 -- 16
CC-0 or CC-1 25 21.3 74.8 -- 29.4
Hall et al. [34] CC-0 2004 34 MMC -- -- -- --
11.2 45
Yonemura et al. [32] CC-0 2005 107 MMC-cisplatin-etoposide 11.5 -- -- 6.5
47 15.5 -- -- 27
Scaringi et al. [41] CC-0 2008 32 MMC-cisplatin 6.6 -- -- --
8 15
Glehen et al. [33] CC-0* 2010 159 Various 9.2 43 18 13
85 15 61 30 23
From Sugarbaker et al. [43] with permission. CC-0: complete macroscopic cytoreduction; CC-1: residual tumor nodules < 5 mm; MMC:
mitomycin C; HIPEC: hyperthermic intraperitoneal chemotherapy
Although there may be a survival benefit, perioperative intraperitoneal chemotherapy can increase
morbidities. Even the most experienced peritonectomy centers that remove all macroscopic disease and
[30]
then administer intraperitoneal chemotherapy have a higher morbidity and cost [32-34] . Yan et al. discussed
an association of improved overall survival with HIPEC with or without EPIC after resection of advanced
gastric primary cancer, however, with EPIC there was an associated greater risk for intraabdominal abscess (P
[35]
= 0.003) and neutropenia (P = 0.007). Yu et al. also saw an increased risk of intra-abdominal abscess with
the use of EPIC compared to the control arm. Intraperitoneal chemotherapy does have less systemic toxicity
as compared to systemic chemotherapy. Although individual studies did not show a significant difference
in neutropenia between treatment arms, the meta-analysis demonstrated a significantly higher risk of
[30]
neutropenia in the intraperitoneal chemotherapy arm .
Most of the randomized studies were completed in Asia and it is unknown if they can be compared
with disease in Western areas. Perioperative chemotherapy may be of greater benefit in Western patients
with more advanced disease and less lymph nodes dissected. Data does suggest a role of HIPEC with or
without EPIC to improve overall survival for advanced primary gastric cancer with advanced T-stage and
no peritoneal metastases. A prospective multi-institutional randomized controlled trial with well-defined
eligibility criteria, interventions and end-points is currently in progress in France (D2 resection ± HIPEC) in
locally advanced gastric carcinoma, GASTRICHIP, ClinicalTrials.gov Identifier: NCT01882933.
TREATMENT PROTOCOLS FOR GASTRIC CANCER WITH PERITONEAL METASTASES
Gastric cancer with peritoneal metastases has been considered a terminal condition. Prospective studies had
[36]
a median survival of less than 6 months . Although response rates to systemic chemotherapy regimens
have improved, there has not been a similar reflection in survival rates . There may be some effective
[37]
palliation of gastric cancer resections in patients with peritoneal metastases, however there is no long-term
improvement in survival.
CYTOREDUCTIVE SURGERY AND HIPEC AS AN EFFECTIVE STRATEGY
There is potential for long-term survival for patients with gastric cancer and peritoneal metastases with
the combined use of CRS and HIPEC. There are single institutional data and phase II studies that support
[33]
use of this strategy [Table 1] [31-34,38-41] . Glehen et al. studied 159 patients with a median follow-up of
20.4 months. There was a median overall survival of 9.2 months but the 5-year survival rate was 13%.
Although CRS and HIPEC in gastric cancer with peritoneal metastases is less effective than with other