Page 959 - Read Online
P. 959
Brodosi et al. Hepatoma Res 2020;6:82 I http://dx.doi.org/10.20517/2394-5079.2020.88 Page 3 of 11
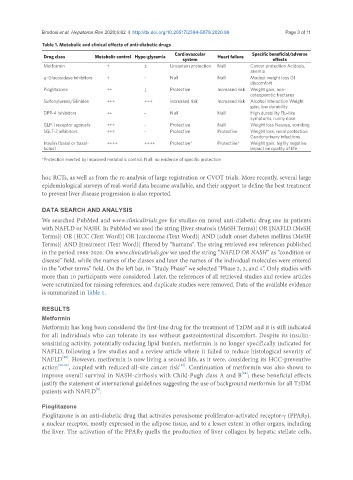
Table 1. Metabolic and clinical effects of anti-diabetic drugs
Cardiovascular Specific beneficial/adverse
Drug class Metabolic control Hypo-glycemia Heart failure
system effects
Metformin + ± Uncertain protection Null Cancer protection Acidosis,
anemia
a-Glucosidase inhibitors + - Null Null Modest weight loss GI
discomfort
Pioglitazone ++ ± Protective Increased risk Weight gain, non-
osteoporotic fractures
Sulfonylureas/Glinides +++ +++ Increased risk Increased risk Alcohol interaction Weight
gain, low durability
DPP-4 inhibitors ++ - Null Null High durability Flu-like
symptoms, runny nose
GLP-1 receptor agonists +++ - Protective Null Weight loss Nausea, vomiting
SGLT-2 inhibitors +++ - Protective Protective Weight loss, renal protection
Genito-urinary infections
Insulin (basal or basal- ++++ ++++ Protective* Protective* Weight gain, highly negative
bolus) impact on quality of life
*Protection exerted by improved metabolic control; Null: no evidence of specific protection
hoc RCTs, as well as from the re-analysis of large registration or CVOT trials. More recently, several large
epidemiological surveys of real-world data became available, and their support to define the best treatment
to prevent liver disease progression is also reported.
DATA SEARCH AND ANALYSIS
We searched PubMed and www.clinicaltrials.gov for studies on novel anti-diabetic drug use in patients
with NAFLD or NASH. In PubMed we used the string [liver steatosis (MeSH Terms)] OR [NAFLD (MeSH
Terms)] OR [HCC (Text Word)] OR [carcinoma (Text Word)] AND [adult onset diabetes mellitus (MeSH
Terms)] AND [treatment (Text Word)] filtered by “humans”. The string retrieved 694 references published
in the period 1988-2020. On www.clinicaltrials.gov we used the string “NAFLD OR NASH” as “condition or
disease” field, while the names of the classes and later the names of the individual molecules were entered
in the “other terms” field. On the left bar, in “Study Phase” we selected “Phase 2, 3, and 4”. Only studies with
more than 10 participants were considered. Later, the references of all retrieved studies and review articles
were scrutinized for missing references, and duplicate studies were removed. Data of the available evidence
is summarized in Table 1.
RESULTS
Metformin
Metformin has long been considered the first-line drug for the treatment of T2DM and it is still indicated
for all individuals who can tolerate its use without gastrointestinal discomfort. Despite its insulin-
sensitizing activity, potentially reducing lipid burden, metformin is no longer specifically indicated for
NAFLD, following a few studies and a review article where it failed to reduce histological severity of
[29]
NAFLD . However, metformin is now living a second life, as it were, considering its HCC-preventive
[33]
action [30-32] , coupled with reduced all-site cancer risk . Continuation of metformin was also shown to
improve overall survival in NASH-cirrhosis with Child-Pugh class A and B ; these beneficial effects
[34]
justify the statement of international guidelines suggesting the use of background metformin for all T2DM
patients with NAFLD .
[8]
Pioglitazone
Pioglitazone is an anti-diabetic drug that activates peroxisome proliferator-activated receptor-γ (PPARγ),
a nuclear receptor, mostly expressed in the adipose tissue, and to a lesser extent in other organs, including
the liver. The activation of the PPARγ quells the production of liver collagen by hepatic stellate cells,