Page 8 - Read Online
P. 8
Pelizzaro et al. Hepatoma Res 2021;7:36 https://dx.doi.org/10.20517/2394-5079.2021.24 Page 3 of 7
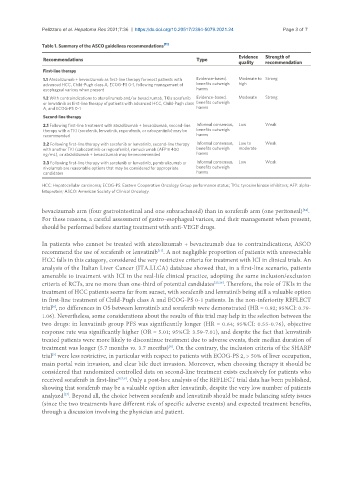
Table 1. Summary of the ASCO guidelines recommendations [11]
Evidence Strength of
Recommendations Type
quality recommendation
First-line therapy
1.1 Atezolizumab + bevacizumab as first-line therapy for most patients with Evidence-based, Moderate to Strong
advanced HCC, Child-Pugh class A, ECOG-PS 0-1, following management of benefits outweigh high
esophageal varices when present harms
1.2 With contraindications to atezolizumab and/or bevacizumab, TKIs sorafenib Evidence-based, Moderate Strong
or lenvatinib as first-line therapy of patients with advanced HCC, Child-Pugh class benefits outweigh
A, and ECOG-PS 0-1 harms
Second-line therapy
2.1 Following first-line treatment with atezolizumab + bevacizumab, second-line Informal consensus, Low Weak
therapy with a TKI (sorafenib, lenvatinib, regorafenib, or cabozantinib) may be benefits outweigh
recommended harms
2.2 Following first-line therapy with sorafenib or lenvatinib, second-line therapy Informal consensus, Low to Weak
with another TKI (cabozantinib or regorafenib), ramucirumab (AFP ≥ 400 benefits outweigh moderate
ng/mL), or atezolizumab + bevacizumab may be recommended harms
2.3 Following first-line therapy with sorafenib or lenvatinib, pembrolizumab or Informal consensus, Low Weak
nivolumab are reasonable options that may be considered for appropriate benefits outweigh
candidates harms
HCC: Hepatocellular carcinoma; ECOG-PS: Eastern Cooperative Oncology Group performance status; TKIs: tyrosine kinase inhibitors; AFP: alpha-
fetoprotein; ASCO: American Society of Clinical Oncology.
[24]
bevacizumab arm (four gastrointestinal and one subarachnoid) than in sorafenib arm (one peritoneal) .
For these reasons, a careful assessment of gastro-esophageal varices, and their management when present,
should be performed before starting treatment with anti-VEGF drugs.
In patients who cannot be treated with atezolizumab + bevacizumab due to contraindications, ASCO
recommend the use of sorafenib or lenvatinib . A not negligible proportion of patients with unresectable
[11]
HCC falls in this category, considered the very restrictive criteria for treatment with ICI in clinical trials. An
analysis of the Italian Liver Cancer (ITA.LI.CA) database showed that, in a first-line scenario, patients
amenable to treatment with ICI in the real-life clinical practice, adopting the same inclusion/exclusion
criteria of RCTs, are no more than one-third of potential candidates [25,26] . Therefore, the role of TKIs in the
treatment of HCC patients seems far from sunset, with sorafenib and lenvatinib being still a valuable option
in first-line treatment of Child-Pugh class A and ECOG-PS 0-1 patients. In the non-inferiority REFLECT
[8]
trial , no differences in OS between lenvatinib and sorafenib were demonstrated (HR = 0.92; 95%CI: 0.79-
1.06). Nevertheless, some considerations about the results of this trial may help in the selection between the
two drugs: in lenvatinib group PFS was significantly longer (HR = 0.64; 95%CI: 0.55-0.76), objective
response rate was significantly higher (OR = 5.01; 95%CI: 3.59-7.01), and despite the fact that lenvatinib
treated patients were more likely to discontinue treatment due to adverse events, their median duration of
treatment was longer (5.7 months vs. 3.7 months) . On the contrary, the inclusion criteria of the SHARP
[8]
trial were less restrictive, in particular with respect to patients with ECOG-PS 2, > 50% of liver occupation,
[3]
main portal vein invasion, and clear bile duct invasion. Moreover, when choosing therapy it should be
considered that randomized controlled data on second-line treatment exists exclusively for patients who
received sorafenib in first-line [6,7,9] . Only a post-hoc analysis of the REFLECT trial data has been published,
showing that sorafenib may be a valuable option after lenvatinib, despite the very low number of patients
analyzed . Beyond all, the choice between sorafenib and lenvatinib should be made balancing safety issues
[27]
(since the two treatments have different risk of specific adverse events) and expected treatment benefits,
through a discussion involving the physician and patient.