Page 45 - Read Online
P. 45
Hewitt et al. Hepatoma Res 2021;7:75 https://dx.doi.org/10.20517/2394-5079.2021.83 Page 9 of 19
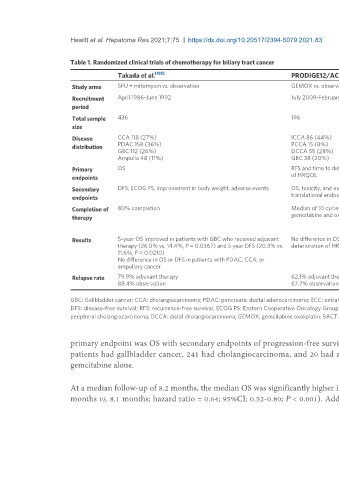
Table 1. Randomized clinical trials of chemotherapy for biliary tract cancer
Takada et al. [125] PRODIGE12/ACCORD18 BILCAP BCAT
Study arms 5FU + mitomycin vs. observation GEMOX vs. observation Capecitabine vs. observation Gemcitabine vs. observation
Recruitment April 1986-June 1992 July 2009-February 2014 March 2006-December 2017 September 2007-January 2011
period
Total sample 436 196 447 225
size
Disease CCA 118 (27%) ICCA 86 (44%) ICCA 84 (19%) PCCA 101
distribution PDAC 158 (36%) PCCA 15 (8%) PCCA 128 (28%) DCCA 124
GBC 112 (26%) DCCA 55 (28%) DCCA 156 (35%)
Ampulla 48 (11%) GBC 38 (20%) GBC 79 (18%)
Primary OS RFS and time to definitive deterioration OS OS
endpoints of HRQOL
Secondary DFS, ECOG PS, improvement in body weight, adverse events OS, toxicity, and exploratory Per-protocol analysis of OS/RFS, RFS, toxicity, RFS and toxicity
endpoints translational endpoint health economics, and quality of life
Completion of 80% completion Median of 10 cycles of 10 for 55% completed chemotherapy, 10 patients (4%) 52.1% completed chemotherapy
therapy gemcitabine and oxaliplatin had 0 cycles, 32% discontinued therapy due to
toxicity 18 patients stopped Gem due to
needing tor dose reduction
Results 5-year OS improved in patients with GBC who received adjuvant No difference in OS, RFS, or No significant difference in OS in intention to treat Gemcitabine provided no
therapy (26.0% vs. 14.4%, P = 0.0367) and 5 year DFS (20.3% vs. deterioration of HRQOL population difference in OS or RFS
11.6%, P = 0.0210) Significant improvement with capecitabine in OS
No difference in OS or DFS in patients with PDAC, CCA, or and RFS in prespecified per-protocol analysis
ampullary cancer
Relapse rate 79.9% adjuvant therapy 62.1% adjuvant therapy 60% adjuvant therapy 53.8% adjuvant therapy
88.4% observation 67.7% observation 65% observation 56.5% observation
GBC: Gallbladder cancer; CCA: cholangiocarcinoma; PDAC: pancreatic ductal adenocarcinoma; ECC: extrahepatic cholangiocarcinoma; ICC: intrahepatic cholangiocarcinoma; 5FU: 5-fluorouracil; OS: overall survival;
DFS: disease-free survival; RFS: recurrence-free survival; ECOG PS: Eastern Cooperative Oncology Group Performance Status; HRQOL: health-related quality of life; ICCA: intrahepatic Cholangiocarcinoma; PCC:
peripheral cholangiocarcinoma; DCCA: distal cholangiocarcinoma; GEMOX: gemcitabine oxaliplatin; BACT: bile duct adjuvant cancer trial.
primary endpoint was OS with secondary endpoints of progression-free survival, tumor response, and adverse events. Of the 410 patients randomized, 149
patients had gallbladder cancer, 241 had cholangiocarcinoma, and 20 had ampullary cancer; 204 received cisplatin plus gemcitabine, and 206 received
gemcitabine alone.
At a median follow-up of 8.2 months, the median OS was significantly higher in patients who received cisplatin-gemcitabine than the gemcitabine alone (11.7
months vs. 8.1 months; hazard ratio = 0.64; 95%CI: 0.52-0.80; P < 0.001). Additionally, patients who received cisplatin-gemcitabine had improved PFS (8.0