Page 128 - Read Online
P. 128
Page 4 of 7 Umar et al. Hepatoma Res 2018;4:71 I http://dx.doi.org/10.20517/2394-5079.2018.31
100
96.1%
90
86.11%
83.33%
80
70
60
50
40
30
20
10
0
RVR ETR SVR
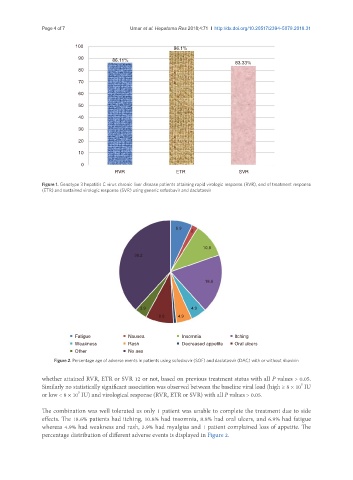
Figure 1. Genotype 3 hepatitis C virus chronic liver disease patients attaining rapid virologic response (RVR), end of treatment response
(ETR) and sustained virologic response (SVR) using generic sofosbuvir and daclatasvir
6.9
2
10.8
38.2
18.6
3.9 4.9
8.8 4.9
1
Fatigue Nausea Insomnia Itching
Weakness Rash Decreased appetite Oral ulcers
Other No aes
Figure 2. Percentage age of adverse events in patients using sofosbuvir (SOF) and daclatasvir (DAC) with or without ribavirin
whether attained RVR, ETR or SVR 12 or not, based on previous treatment status with all P values > 0.05.
5
Similarly no statistically significant association was observed between the baseline viral load (high ≥ 8 × 10 IU
5
or low < 8 × 10 IU) and virological response (RVR, ETR or SVR) with all P values > 0.05.
The combination was well tolerated as only 1 patient was unable to complete the treatment due to side
effects. The 18.6% patients had itching, 10.8% had insomnia, 8.8% had oral ulcers, and 6.9% had fatigue
whereas 4.9% had weakness and rash, 3.9% had myalgias and 1 patient complained loss of appetite. The
percentage distribution of different adverse events is displayed in Figure 2.