Page 29 - Read Online
P. 29
Missale et al. Hepatoma Res 2018;4:22 I http://dx.doi.org/10.20517/2394-5079.2018.72 Page 7 of 15
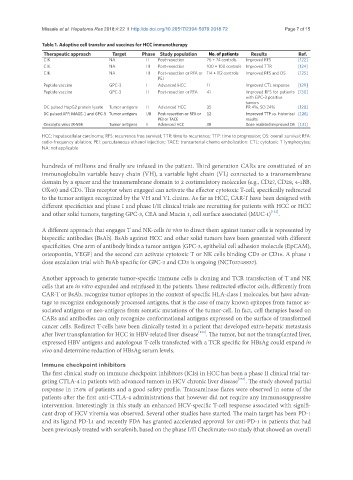
Table 1. Adoptive cell transfer and vaccines for HCC immunotherapy
Therapeutic approach Target Phase Study population No. of patients Results Ref.
CIK NA II Post-resection 76 + 74 controls Improved RFS [122]
CIK NA III Post-resection 100 + 100 controls Improved TTR [124]
CIK NA III Post-resection or RFA or 114 + 112 controls Improved RFS and OS [125]
PEI
Peptide vaccine GPC-3 I Advanced HCC 11 Improved CTL response [129]
Peptide vaccine GPC-3 II Post-resection or RFA 41 Improved RFS for patients [130]
with GPC-3 positive
tumors
DC pulsed HepG2 protein lysate Tumor antigens II Advanced HCC 35 PR 4%, SD 24% [128]
DC pulsed AFP, MAGE-1 and GPC-3 Tumor antigens I/II Post-resection or RFA or 12 Improved TTP vs. historical [126]
PEI or TACE results
Oncolytic virus JX-594 Tumor antigens II Advanced HCC 30 Dose realated improved OS [131]
HCC: hepatocellular carcinoma; RFS: recurrence free survival; TTR: time to recurrence; TTP: time to progression; OS: overall survival; RFA:
radio-frequency ablation; PEI: percutaneous ethanol injection; TACE: transarterial chemo embolization: CTL: cytotoxic T lymphocytes;
NA: not applicable
hundreds of millions and finally are infused in the patient. Third generation CARs are constituted of an
immunoglobulin variable heavy chain (VH), a variable light chain (VL) connected to a transmembrane
domain by a spacer and the transmembrane domain to 2 costimulatory molecules (e.g., CD27, CD28, 4-1BB,
OX40) and CD3. This receptor when engaged can activate the effector cytotoxic T-cell, specifically redirected
to the tumor antigen recognized by the VH and VL chains. As far as HCC, CAR-T have been designed with
different specificities and phase I and phase I/II clinical trials are recruiting for patients with HCC or HCC
and other solid tumors, targeting GPC-3, CEA and Mucin 1, cell surface associated (MUC-1) [132] .
A different approach that engages T and NK-cells in vivo to direct them against tumor cells is represented by
bispecific antibodies (BsAb). BsAb against HCC and other solid tumors have been generated with different
specificities. One arm of antibody binds a tumor antigen [GPC-3, epithelial cell adhesion molecule (EpCAM),
osteopontin, VEGF] and the second can activate cytotoxic T or NK cells binding CD3 or CD16. A phase 1
dose escalation trial with BsAb specific for GPC-3 and CD3 is ongoing (NCT02748837).
Another approach to generate tumor-specific immune cells is cloning and TCR transfection of T and NK
cells that are in vitro expanded and reinfused in the patients. These redirected effector cells, differently from
CAR-T or BsAb, recognize tumor epitopes in the context of specific HLA-class I molecules, but have advan-
tage to recognize endogenously processed antigens, that is the case of many known epitopes from tumor as-
sociated antigens or neo-antigens from somatic mutations of the tumor-cell. In fact, cell therapies based on
CARs and antibodies can only recognize conformational antigens expressed on the surface of transformed
cancer cells. Redirect T-cells have been clinically tested in a patient that developed extra-hepatic metastasis
after liver transplantation for HCC in HBV-related liver disease [133] . The tumor, but not the transplanted liver,
expressed HBV antigens and autologous T-cells transfected with a TCR specific for HBsAg could expand in
vivo and determine reduction of HBsAg serum levels.
Immune checkpoint inhibitors
The first clinical study on immune checkpoint inhibitors (ICIs) in HCC has been a phase II clinical trial tar-
geting CTLA-4 in patients with advanced tumors in HCV chronic liver disease [134] . The study showed partial
response in 17.6% of patients and a good safety profile. Transaminase flares were observed in some of the
patients after the first anti-CTLA-4 administrations that however did not require any immunosuppressive
intervention. Interestingly in this study an enhanced HCV-specific T-cell response associated with signifi-
cant drop of HCV viremia was observed. Several other studies have started. The main target has been PD-1
and its ligand PD-L1 and recently FDA has granted accelerated approval for anti-PD-1 in patients that had
been previously treated with sorafenib, based on the phase I/II Checkmate-040 study (that showed an overall