Page 19 - Read Online
P. 19
Hafezi et al. Hepatoma Res 2018;4:16 I http://dx.doi.org/10.20517/2394-5079.2018.55 Page 3 of 8
HBV-HCC patient Engineering of HBV-specific TCR-redirected T cells
PBMC isolation In vitro T cells activation & expansion
Modification of T cells
Blood apheresis a b Viral vectors Electroporation
CD4
TCR
mRNA
CD8
MHC-dextramer
TCR
HBV-specific redirected T cells Peptide
Viral vector mRNA
Electroporation
MHC + peptide
Translation Granules
Hepatocyte
HBV infected HBV-HCC Reinfusion Quality control TCR-engineered T cells HBV virus
Cell death Cell survival Tumor
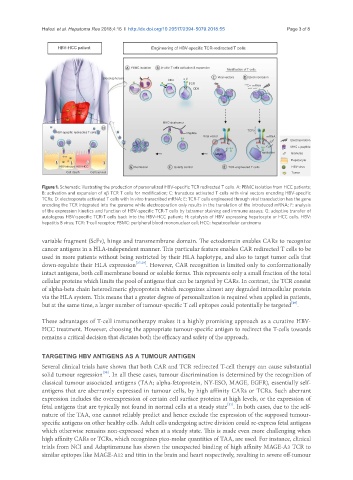
Figure 1. Schematic illustrating the production of personalized HBV-specific TCR redirected T cells. A: PBMC isolation from HCC patients;
B: activation and expansion of αβ TCR T cells for modification; C: transduce activated T cells with viral vectors encoding HBV-specific
TCRs; D: electroporate activated T cells with in vitro transcribed mRNA; E: TCR-T cells engineered through viral transduction has the gene
encoding the TCR integrated into the genome while electroporation only results in the translation of the introduced mRNA; F: analysis
of the expression kinetics and function of HBV-specific TCR-T cells by tetramer staining and immune assays; G: adoptive transfer of
autologous HBV-specific TCR-T cells back into the HBV-HCC patient; H: cytolysis of HBV expressing hepatocyte or HCC cells. HBV:
hepatitis B virus; TCR: T-cell receptor; PBMC: peripheral blood mononuclear cell; HCC: hepatocellular carcinoma
variable fragment (ScFv), hinge and transmembrane domain. The ectodomain enables CARs to recognize
cancer antigens in a HLA-independent manner. This particular feature enables CAR redirected T cells to be
used in more patients without being restricted by their HLA haplotype, and also to target tumor cells that
down-regulate their HLA expression [27,28] . However, CAR recognition is limited only to conformationally
intact antigens, both cell membrane bound or soluble forms. This represents only a small fraction of the total
cellular proteins which limits the pool of antigens that can be targeted by CARs. In contrast, the TCR consist
of alpha-beta chain heterodimeric glycoprotein which recognizes almost any degraded intracellular protein
via the HLA system. This means that a greater degree of personalization is required when applied in patients,
[29]
but at the same time, a larger number of tumour-specific T cell epitopes could potentially be targeted .
These advantages of T-cell immunotherapy makes it a highly promising approach as a curative HBV-
HCC treatment. However, choosing the appropriate tumour-specific antigen to redirect the T-cells towards
remains a critical decision that dictates both the efficacy and safety of the approach.
TARGETING HBV ANTIGENS AS A TUMOUR ANTIGEN
Several clinical trials have shown that both CAR and TCR redirected T-cell therapy can cause substantial
[30]
solid tumour regression . In all these cases, tumour discrimination is determined by the recognition of
classical tumour associated antigens (TAA; alpha-fetoprotein, NY-ESO, MAGE, EGFR), essentially self-
antigens that are aberrantly expressed in tumour cells, by high affinity CARs or TCRs. Such aberrant
expression includes the overexpression of certain cell surface proteins at high levels, or the expression of
[31]
fetal antigens that are typically not found in normal cells at a steady state . In both cases, due to the self-
nature of the TAA, one cannot reliably predict and hence exclude the expression of the supposed tumour-
specific antigens on other healthy cells. Adult cells undergoing active division could re-express fetal antigens
which otherwise remains non-expressed when at a steady state. This is made even more challenging when
high affinity CARs or TCRs, which recognizes pico-molar quantities of TAA, are used. For instance, clinical
trials from NCI and Adaptimmune has shown the unexpected binding of high affinity MAGE-A3 TCR to
similar epitopes like MAGE-A12 and titin in the brain and heart respectively, resulting in severe off-tumour