Page 20 - Read Online
P. 20
Page 20 Kaehler et al. Cancer Drug Resist 2019;2:18-30 I http://dx.doi.org/10.20517/cdr.2019.05
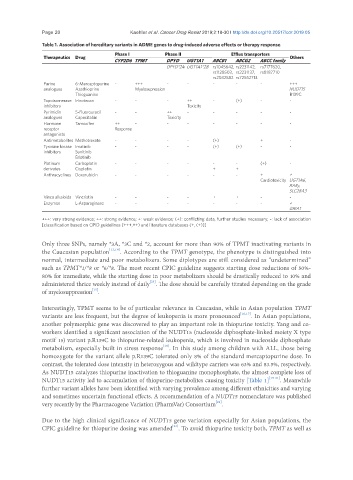
Table 1. Association of hereditary variants in ADME genes to drug-induced adverse effects or therapy response
Phase I Phase II Efflux transporters
Therapeutics Drug Others
CYP2D6 TPMT DPYD UGT1A1 ABCB1 ABCG2 ABCC family
DPYD*2A UGT1A1*28 rs1045642, rs2231142, rs7177620,
rs1128503, rs2231137, rs8187710
rs2032582 rs72552713
Purine 6-Mercaptopurine - +++ - - - - - +++
analogues Azathioprine Myelosupression NUDT15
Thioguanine R139C
Topoisomerase Irinotecan - - - ++ - (+) - -
inhibitors Toxicity
Pyrimidin 5-Fluorouracil - - ++ - - - - -
analogues Capecitabin Toxicity
Hormone Tamoxifen ++ - - - - - - -
receptor Response
antagonists
Antimetabolites Methotrexate - - - - (+) + -
Tyrosine kinase Imatinib - - - - (+) (+) - -
inhibitors Sunitinib
Erlotinib
Platinum Carboplatin - - - - - - (+) -
derivates Cisplatin + +
Anthracyclines Doxorubicin - - - - - - + +
Cardiotoxicity UGT1A6,
RARγ,
SLC28A3
Vinca alkaloids Vincristin - - - - + + - -
Enzymes L-Asparaginase - - - - - - - +
GRIA1
+++: very strong evidence; ++: strong evidence; +: weak evidence; (+): conflicting data, further studies necessary; -: lack of association
[classification based on CPID guidelines (+++,++) and literature databases (+, (+))]
Only three SNPs, namely *3A, *3C and *2, account for more than 90% of TPMT inactivating variants in
the Caucasian population [13,14] . According to the TPMT genotype, the phenotype is distinguished into
normal, intermediate and poor metabolizers. Some diplotypes are still considered as “undetermined”
such as TPMT*1/*8 or *6/*8. The most recent CPIC guideline suggests starting dose reductions of 50%-
80% for immediate, while the starting dose in poor metabolizers should be drastically reduced to 10% and
[23]
administered thrice weekly instead of daily . The dose should be carefully titrated depending on the grade
[15]
of myelosuppression .
Interestingly, TPMT seems to be of particular relevance in Caucasian, while in Asian population TPMT
variants are less frequent, but the degree of leukopenia is more pronounced [16,17] . In Asian populations,
another polymorphic gene was discovered to play an important role in thiopurine toxicity. Yang and co-
workers identified a significant association of the NUDT15 (nucleoside diphosphate-linked moiety X type
motif 15) variant p.R139C to thiopurine-related leukopenia, which is involved in nucleoside diphosphate
metabolism, especially built in stress response . In this study among children with ALL, those being
[18]
homozygote for the variant allele p.R139C tolerated only 8% of the standard mercaptopurine dose. In
contrast, the tolerated dose intensity in heterozygous and wildtype carriers was 63% and 83.5%, respectively.
As NUDT15 catalyzes thiopurine inactivation to thioguanine monophosphate, the almost complete loss of
NUDT15 activity led to accumulation of thiopurine-metabolites causing toxicity [Table 1] [19-21] . Meanwhile
further variant alleles have been identified with varying prevalence among different ethnicities and varying
and sometimes uncertain functional effects. A recommendation of a NUDT15 nomenclature was published
[22]
very recently by the Pharmacogene Variation (PharmVar) Consortium .
Due to the high clinical significance of NUDT15 gene variation especially for Asian populations, the
CPIC guideline for thiopurine dosing was amended . To avoid thiopurine toxicity both, TPMT as well as
[23]