Page 180 - Read Online
P. 180
Page 112 Ravegnini et al. Cancer Drug Resist 2019;2:107-15 I http://dx.doi.org/10.20517/cdr.2019.02
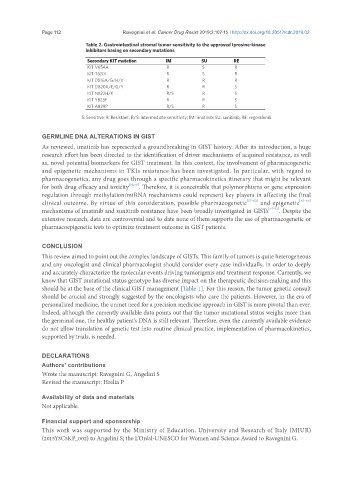
Table 2. Gastrointestinal stromal tumor sensitivity to the approved tyrosine-kinase
inhibitors basing on secondary mutations
Secondary KIT mutation IM SU RE
KIT V654A R S R
KIT T670I R S R
KIT D816A/G/H/V R R R
KIT D820A/E/G/Y R R S
KIT N822H/K R/S R S
KIT Y823F R R S
KIT A829P R/S R S
S: Sensitive; R: Resistant; R/S: intermediate sensitivity; IM: imatinib; SU: sunitinib; RE: regorafenib
GERMLINE DNA ALTERATIONS IN GIST
As reviewed, imatinib has represented a groundbreaking in GIST history. After its introduction, a huge
research effort has been directed to the identification of driver mechanisms of acquired resistance, as well
as, novel potential biomarkers for GIST treatment. In this context, the involvement of pharmacogenetic
and epigenetic mechanisms in TKIs resistance has been investigated. In particular, with regard to
pharmacogenetics, any drug goes through a specific pharmacokinetics itinerary that might be relevant
for both drug efficacy and toxicity [55,56] . Therefore, it is conceivable that polymorphisms or gene expression
regulation through methylation/miRNA mechanisms could represent key players in affecting the final
clinical outcome. By virtue of this consideration, possible pharmacogenetic [57-62] and epigenetic [63-66]
mechanisms of imatinib and sunitinib resistance have been broadly investigated in GISTs [67-71] . Despite the
extensive research, data are controversial and to date none of them supports the use of pharmacogenetic or
pharmacoepigenetic tests to optimize treatment outcome in GIST patients.
CONCLUSION
This review aimed to point out the complex landscape of GISTs. This family of tumors is quite heterogeneous
and any oncologist and clinical pharmacologist should consider every case individually, in order to deeply
and accurately characterize the molecular events driving tumorigenis and treatment response. Currently, we
know that GIST mutational status genotype has diverse impact on the therapeutic decision-making and this
should be at the base of the clinical GIST management [Table 1]. For this reason, the tumor genetic consult
should be crucial and strongly suggested by the oncologists who care the patients. However, in the era of
personalized medicine, the unmet need for a precision medicine approach in GIST is more pivotal than ever.
Indeed, although the currently available data points out that the tumor mutational status weighs more than
the germinal one, the healthy patient’s DNA is still relevant. Therefore, even the currently available evidence
do not allow translation of genetic test into routine clinical practice, implementation of pharmacokinetics,
supported by trials, is needed.
DECLARATIONS
Authors’ contributions
Wrote the manuscript: Ravegnini G, Angelini S
Revised the manuscript: Hrelia P
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Ministry of Education, University and Research of Italy (MIUR)
(2015Y3C5KP_002) to Angelini S; the L’Oréal-UNESCO for Women and Science Award to Ravegnini G.