Page 30 - Read Online
P. 30
Nguyen et al. Cancer Drug Resist 2018;1:126-38 I http://dx.doi.org/10.20517/cdr.2018.08 Page 129
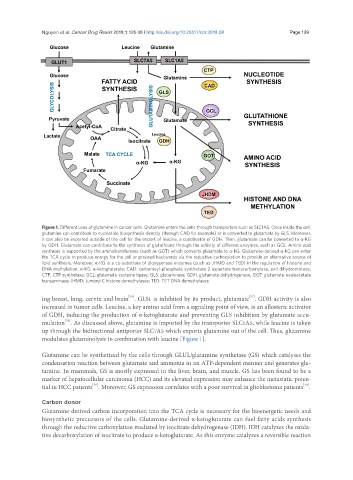
Figure 1. Different uses of glutamine in cancer cells. Glutamine enters the cells through transporters such as SLC1A5. Once inside the cell,
glutamine can contribute to nucleotide biosynthesis directly (through CAD for example) or is converted to glutamate by GLS. Moreover,
it can also be exported outside of the cell for the import of leucine, a coactivator of GDH. Then, glutamate can be converted to α-KG
by GDH. Glutamate can contribute to the synthesis of glutathione through the activity of different enzymes, such as GCL. Amino acid
synthesis is supported by the aminotransferases (such as GOT) which converts glutamate to α-KG. Glutamine-derived α-KG can enter
the TCA cycle to produce energy for the cell or proceed backwards via the reductive carboxylation to provide an alternative source of
lipid synthesis. Moreover, α-KG is a co-substrate of dioxygenase enzymes (such as JHMD and TED) in the regulation of histone and
DNA methylation. α-KG: α-ketoglutarate; CAD: carbamoyl-phosphate synthetase 2 aspartate transcarbamylase, and dihydroorotase;
CTP: CTP synthetase; GCL: glutamate-cysteine ligase; GLS: glutaminase; GDH: glutamate dehydrogenase; GOT: glutamate-oxaloacetate
transaminase; JHMD: Jumonji C histone demethylases; TED: TET DNA demethylases
[36]
[37]
ing breast, lung, cervix and brain . GLS1 is inhibited by its product, glutamate . GDH activity is also
increased in tumor cells. Leucine, a key amino acid from a signaling point of view, is an allosteric activator
of GDH, inducing the production of α-ketoglutarate and preventing GLS inhibition by glutamate accu-
[38]
mulation . As discussed above, glutamine is imported by the transporter SLC1A5, while leucine is taken
up through the bidirectional antiporter SLC7A5 which exports glutamine out of the cell. Thus, glutamine
modulates glutaminolysis in combination with leucine [Figure 1].
Glutamine can be synthetized by the cells through GLUL/glutamine synthetase (GS) which catalyses the
condensation reaction between glutamate and ammonia in an ATP-dependent manner and generates glu-
tamine. In mammals, GS is mostly expressed in the liver, brain, and muscle. GS has been found to be a
marker of hepatocellular carcinoma (HCC) and its elevated expression may enhance the metastatic poten-
[40]
[39]
tial in HCC patients . Moreover, GS expression correlates with a poor survival in glioblastoma patients .
Carbon donor
Glutamine-derived carbon incorporation into the TCA cycle is necessary for the bioenergetic needs and
biosynthetic precursors of the cells. Glutamine-derived α-ketoglutarate can fuel fatty acids synthesis
through the reductive carboxylation mediated by isocitrate dehydrogenase (IDH). IDH catalyzes the oxida-
tive decarboxylation of isocitrate to produce α-ketoglutarate. As this enzyme catalyzes a reversible reaction