Page 13 - Read Online
P. 13
Page 4 of 13 Li et al. Ageing Neur Dis 2022;2:12 https://dx.doi.org/10.20517/and.2022.14
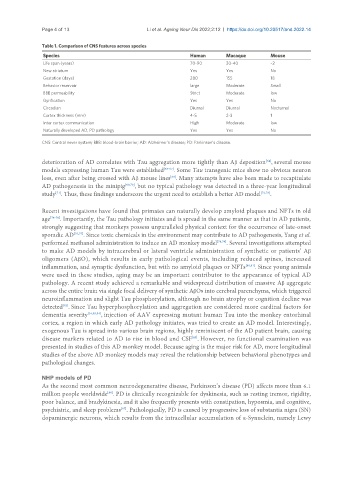
Table 1. Comparison of CNS features across species
Species Human Macaque Mouse
Life span (years) 70-90 30-40 ~2
New striatum Yes Yes No
Gestation (days) 280 155 18
Behavior reservoir large Moderate Small
BBB permeability Strict Moderate low
Gyrification Yes Yes No
Circadian Diurnal Diurnal Nocturnal
Cortex thickness (mm) 4-5 2-3 1
Inter cortex communication High Moderate low
Naturally developed AD, PD pathology Yes Yes No
CNS: Central never system; BBB: blood-brain barrier; AD: Alzheimer’s disease; PD: Parkinson’s disease.
deterioration of AD correlates with Tau aggregation more tightly than Aβ deposition , several mouse
[64]
models expressing human Tau were established [65-67] . Some Tau transgenic mice show no obvious neuron
loss, even after being crossed with Aβ mouse lines . Many attempts have also been made to recapitulate
[68]
AD pathogenesis in the minipig [69,70] , but no typical pathology was detected in a three-year longitudinal
study . Thus, these findings underscore the urgent need to establish a better AD model [72,73] .
[71]
Recent investigations have found that primates can naturally develop amyloid plaques and NFTs in old
age [74-76] . Importantly, the Tau pathology initiates and is spread in the same manner as that in AD patients,
strongly suggesting that monkeys possess unparalleled physical context for the occurrence of late-onset
sporadic AD [75,77] . Since toxic chemicals in the environment may contribute to AD pathogenesis, Yang et al.
performed methanol administration to induce an AD monkey model [78,79] . Several investigations attempted
to make AD models by intracerebral or lateral ventricle administration of synthetic or patients’ Aβ
oligomers (AβO), which results in early pathological events, including reduced spines, increased
inflammation, and synaptic dysfunction, but with no amyloid plaques or NFTs [80,81] . Since young animals
were used in these studies, aging may be an important contributor to the appearance of typical AD
pathology. A recent study achieved a remarkable and widespread distribution of massive Aβ aggregate
across the entire brain via single focal delivery of synthetic AβOs into cerebral parenchyma, which triggered
neuroinflammation and slight Tau phosphorylation, although no brain atrophy or cognition decline was
detected . Since Tau hyperphosphorylation and aggregation are considered more cardinal factors for
[82]
dementia severity [54,83,84] , injection of AAV expressing mutant human Tau into the monkey entorhinal
cortex, a region in which early AD pathology initiates, was tried to create an AD model. Interestingly,
exogenous Tau is spread into various brain regions, highly reminiscent of the AD patient brain, causing
[85]
disease markers related to AD to rise in blood and CSF . However, no functional examination was
presented in studies of this AD monkey model. Because aging is the major risk for AD, more longitudinal
studies of the above AD monkey models may reveal the relationship between behavioral phenotypes and
pathological changes.
NHP models of PD
As the second most common neurodegenerative disease, Parkinson’s disease (PD) affects more than 6.1
million people worldwide . PD is clinically recognizable for dyskinesia, such as resting tremor, rigidity,
[86]
poor balance, and bradykinesia, and it also frequently presents with constipation, hyposmia, and cognitive,
psychiatric, and sleep problems . Pathologically, PD is caused by progressive loss of substantia nigra (SN)
[87]
dopaminergic neurons, which results from the intracellular accumulation of α-Synuclein, namely Lewy